A kind of preparation method of 5-amino-2,4,6-triiodoisophthalic acid
A technology of triiodoisophthalic acid and aminoisophthalic acid, which is applied in the field of preparation of 5-amino-2,4,6-triiodoisophthalic acid, can solve difficult operation, many solid wastes and instability and other problems, to achieve the effect of reducing the pressure of water treatment, less solid waste and ideal yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
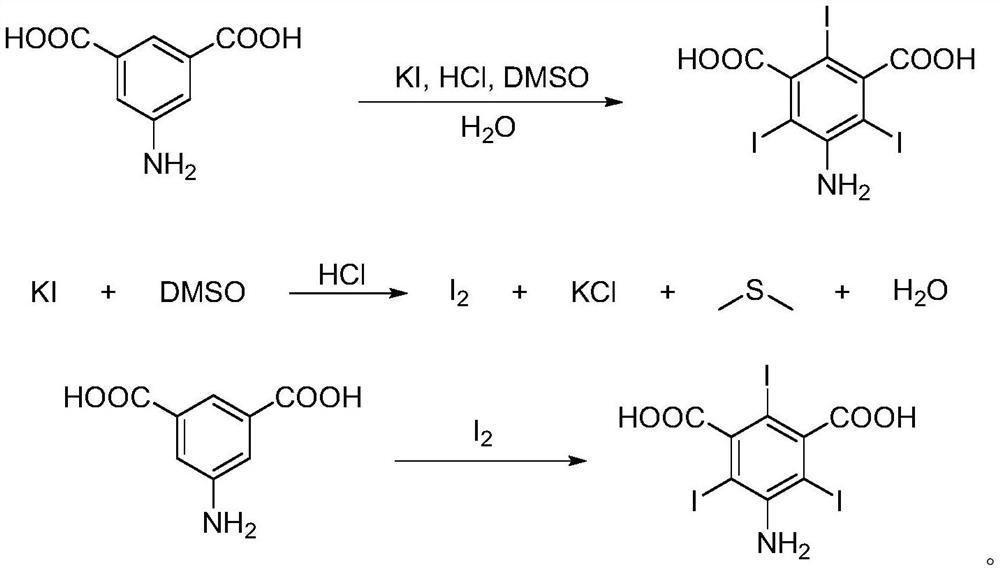
[0022] This example provides a preparation method of 5-amino-2,4,6-triiodoisophthalic acid, the reaction route and principle are as follows:
[0023]
[0024] The preparation method of described 5-amino-2,4,6-triiodoisophthalic acid comprises the steps:
[0025] Add 10mmol 5-aminoisophthalic acid (1.79g), 60mmol KI (9.96g), 60mmol DMSO (3.12g), 90mmol HCl (7.5mL concentrated hydrochloric acid (mass fraction of hydrogen chloride is about 37%)) to 3000mmol water (54 mL), stirred at room temperature for 10 min. The temperature was raised to 100°C to continue the reaction for 16h. After the reaction was completed, it was lowered to room temperature, filtered, and the obtained solid was dissolved in 1M KOH (30 mL), and treated with activated carbon for decolorization. Then add 1M HCl to neutralize to acidity, filter, pickle (1M HCl 10mL×3), and dry to obtain the final solid product 5-amino-2,4,6-triiodoisophthalic acid with a yield of 70%. 95% purity.
Embodiment 2
[0027] This example provides a kind of preparation method of 5-amino-2,4,6-triiodoisophthalic acid, described preparation method comprises the following steps: 10mmol 5-aminoisophthalic acid (1.79g), 60mmol NaI ( 9g), 60mmol DMSO (3.12g), 90mmol HCl (7.5mL concentrated hydrochloric acid) were added to 3000mmol water (54mL), and stirred at room temperature for 10min. The temperature was raised to reflux to continue the reaction for 16h. After the reaction was completed, it was lowered to room temperature, filtered, and the resulting solid was dissolved in 1M KOH (30 mL), and treated with activated carbon for decolorization. Then add 1M HCl to neutralize to acidity, filter, pickle (1M HCl 10mL×3), and dry to obtain the final solid product 5-amino-2,4,6-triiodoisophthalic acid with a yield of 68%. 91% purity.
Embodiment 3
[0029] This example provides a preparation method of 5-amino-2,4,6-triiodoisophthalic acid, the preparation method comprising the following steps: 10mmol 5-aminoisophthalic acid (1.79g), 60mmol NH 4 I (8.7g), 60mmol DMSO (3.12g), 90mmol HCl (7.5mL concentrated hydrochloric acid) were added to 3000mmol water (54mL), and stirred at room temperature for 10min. The temperature was raised to reflux to continue the reaction for 16h. After the reaction was completed, it was lowered to room temperature, filtered, and the resulting solid was dissolved in 1M KOH (30 mL), and treated with activated carbon for decolorization. Then add 1M HCl to neutralize to acidity, filter, pickle (1M HCl 10mL×3), and dry to obtain the final solid product 5-amino-2,4,6-triiodoisophthalic acid with a yield of 73%. 90% purity.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com