Preparation method of high-entropy oxide with high solar energy absorptivity and infrared emissivity
A technology of infrared emissivity and solar energy, which is applied in the field of infrared radiation materials and solar energy absorption materials, can solve the problems of long heat preservation time, high cost of raw materials, and many preparation steps, and achieve high production efficiency, stable crystal structure, and simple preparation technology Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
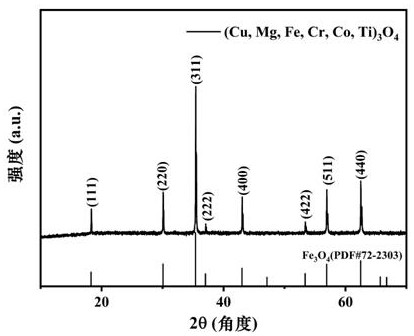
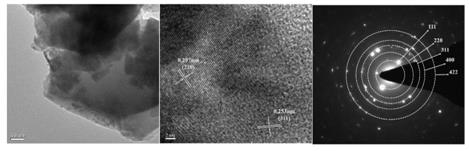
[0034] Weigh CuO 8.751g (0.11mol), MgO4.433g (0.11mol), Fe 2 o 3 8.783g (0.055mol), Cr 2 o 3 8.359g (0.055mol), Co 3 o 4 8.829g (0.0367mol), TiO 28.789g (0.11mol) powder; according to the mass ratio of ball: material: water 3:1:3, pour the ball mill beads, raw materials and ultrapure water into the ball mill jar; and place the ball mill jar on the ball mill station for 1 hour Ball milling at a speed of 450r / min, followed by a pause of 10min, as a ball milling cycle, after 10min, ball milling for 1 hour at a speed of 450r / min, and a total of 7 hours of ball milling to obtain a mixed powder; then the mixed powder was dried and ground; The ground mixture powder was placed in a box-type resistance furnace, heated to 680 °C in an air atmosphere (heating rate of 12 °C / min), calcined for 2 hours, and then cooled to room temperature by air quenching to obtain a single-phase (Cu, Mg, Fe, Cr, Co, Ti) 3 o 4 High entropy oxides.
[0035] figure 1 As prepared (Cu, Mg, Fe, Cr, Co,...
Embodiment 2
[0042] Weigh CuO8.751g (0.11mol), Co 3 o 4 8.829g (0.0367mol), Fe 2 o 3 8.783g (0.055mol), Cr 2 o 3 8.359g (0.055mol), ZnO8.952g (0.11mol), TiO 2 8.789g (0.11mol) powder; according to the mass ratio of ball: material: water 2:1:3, pour the ball mill beads, raw materials and ultrapure water into the ball mill jar; and place the ball mill jar on the ball mill station for 1 hour Ball milling at a speed of 300r / min, followed by a pause of 10min, as a ball milling cycle, after 10min, ball milling for 1 hour at a speed of 300r / min, and a total of 5 hours of ball milling to obtain a mixed powder; then the mixed powder was dried and ground; In a box-type resistance furnace, the temperature was raised to 500°C in an air atmosphere (the heating rate was 10°C / min), calcined for 1 hour, and then cooled to room temperature with the furnace to obtain single-phase (Cu, Co, Fe, Cr, Zn, Ti ) 3 o 4 High entropy oxides.
[0043] Figure 7 As prepared (Cu, Co, Fe, Cr, Zn, Ti) 3 o 4 XR...
Embodiment 3
[0046] Weigh CuO8.751g (0.11mol), MgO4.433g (0.11mol), Fe 2 o 3 8.783g (0.055mol), Cr 2 o 3 8.359g (0.055mol), Co 3 o 4 8.829g (0.0367mol), ZnO8.952g (0.11mol) powder; according to the ball: material: water mass ratio 4:1:3, pour the ball mill beads, raw materials and ultrapure water into the ball mill jar; and place the ball mill jar in the ball mill At the station, ball mill for 1 hour at a speed of 400r / min, and then pause for 10 minutes as a ball milling cycle. After 10 minutes, ball mill for 1 hour at a speed of 400r / min for a total of 9 hours to obtain a mixed powder; The mixed powder was dried and ground; then the mixed powder was placed in a box-type resistance furnace, and the temperature was raised to 780°C in an air atmosphere (the heating rate was 14°C / min), calcined for 4 hours, and then quenched with liquid nitrogen and cooled to room temperature to obtain a single phase (Cu, Mg, Fe, Cr, Co, Zn) 3 o 4 High entropy oxides.
[0047] Figure 8 For prepared ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap