Compositions and methods for t cell engineering
An engineering, cell-based technology, applied in the field of compositions and methods for T cell engineering, capable of solving the problems of time and cost constraints for manufacturing patient-specific T cell products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
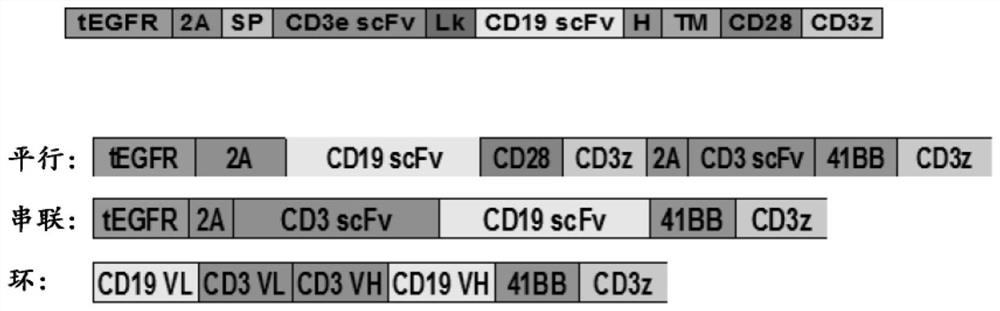
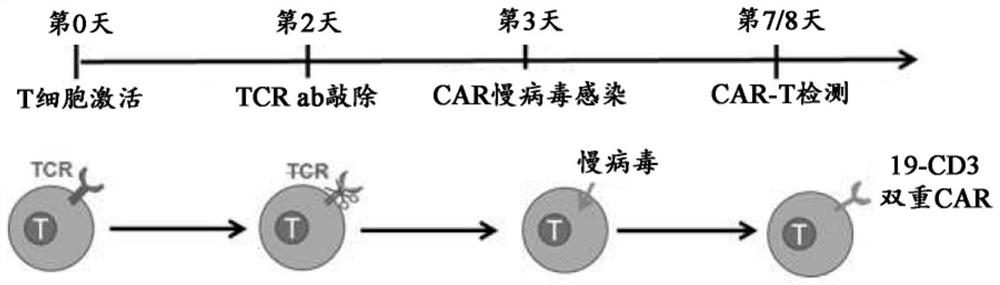
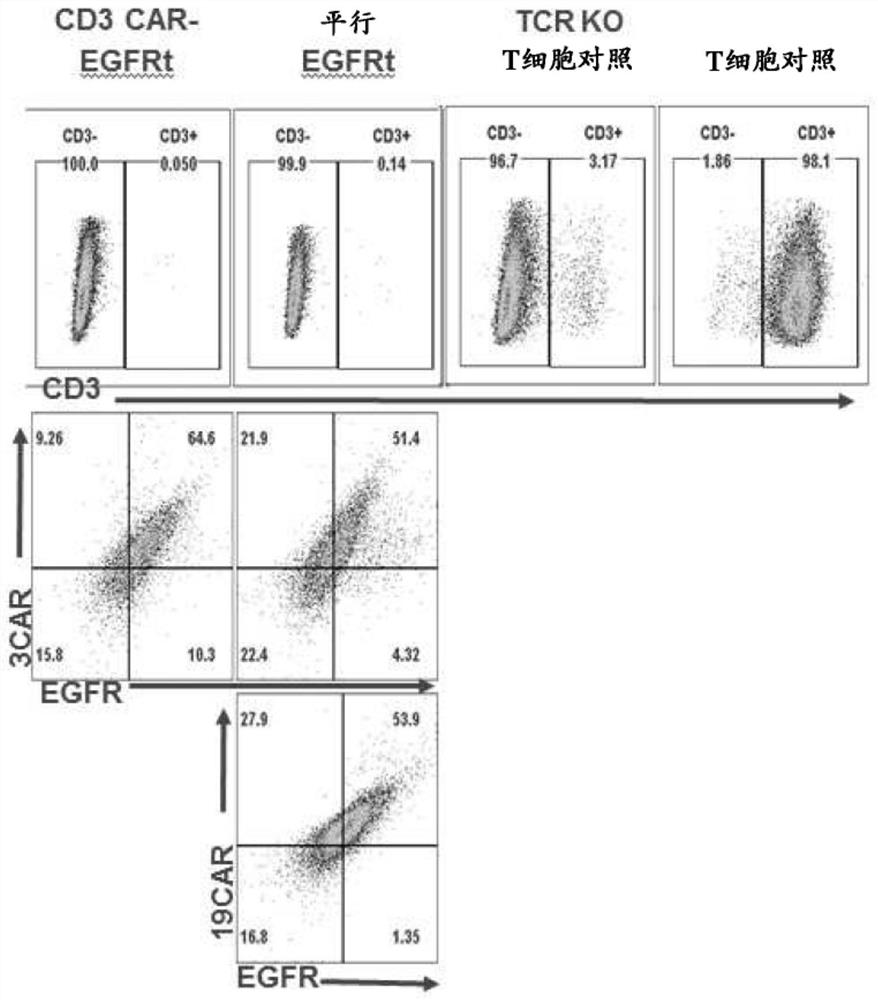
[0303] Example 1 Study of U-CAR T cells expressing CD3+CD19 dual CAR and EGFRt switch with TCR knockout
[0304] General Materials and Methods
[0305] Isolation of peripheral blood mononuclear cells (PBMC) and expansion of T cells from donor blood
[0306] Peripheral blood mononuclear cells (PBMCs) were isolated from donor blood by density gradient centrifugation using Histopaque-1077 (Sigma-Aldrich). T cells are then enriched, activated, cultured and expanded by magnetic beads coupled to anti-CD3 / anti-CD28.
[0307] Culture of cell lines and PBMCs
[0308] Raji cells (Burkitt lymphoma cells, ATCC-CCL86);
[0309] K562 cells (human erythroleukemia cell line, ATCC-CCL243);
[0310] Raji-ffluc cell line (obtained by screening Raji cells transfected with lentivirus with firefly luciferase);
[0311] 293T cells (ATCC-CRL3216).
[0312] Raji cells, K562 cells and Raji-ffluc cell line were cultured in RPMI1640 medium, and 293T cells were cultured in DMEM medium. Both RPMI1...
Embodiment 2
[0370] Example 2 Study of U-CAR-T cells expressing CAR19 and enhancer with TCR knockout
[0371] In this example, 5 x 10 5 Raji luciferase cells were transplanted into NOG mice and grown until reaching 100mm 3 . Will 1×10 6 Wild-type or TCR KO CD19 CAR-T cells were infused intravenously (IV) into Raji-transplanted mice. Tumor burden was assessed by caliper measurement of actual tumor size. The expansion of CAR-T cells in peripheral blood was measured by flow cytometry analysis. Figure 8A Tumor burden after CAR-T treatment is shown. TCR intact CD19 CAR-T cells were able to eliminate tumors, but TCR KO CD19 CAR-T could not control Raji tumor growth. Although both are based on Raji's tumor-bearing model, the subcutaneous model produces solid tumors and has higher requirements for tumor-controlled CAR-T cell proliferation than the intravenous model. Figure 8B CAR-T proliferation in peripheral blood is shown. Compared with WT CAR-T cells, TCR KOCAR-T cells showed prolif...
Embodiment 3
[0375] Example 3 has TCR and CD 7 Double-knockout U-CAR-T cells expressing CAR7 and enhancers, and TCR and CD 2 Double knockout expression of CD2 CAR and enhancer U-CAR-T cell research
[0376] General Materials and Methods
[0377] Isolation of peripheral blood mononuclear cells (PBMC) and expansion of T cells from donor blood
[0378] Peripheral blood mononuclear cells (PBMC) were isolated from donor blood by density gradient centrifugation using Histopaque-1077 (Sigma-Aldrich). T cells are then enriched, activated, cultured and expanded by magnetic beads coupled to anti-CD3 / anti-CD28.
[0379] Cell Lines and T Cell Culture
[0380] CCRF-CEM (T lymphoblastoid cells, ) TM
[0381] CCRF-CEM-luc cells (obtained by screening CCRF-CEM cells transfected with lentivirus with firefly luciferase);
[0382] K562 cells (human erythroleukemia cell line, ATCC-CCL243);
[0383] 293T cells (ATCC-CRL3216);
[0384] NK92 cells (ATCC-CRL2407).
[0385] The CCRF-CEM-luc c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com