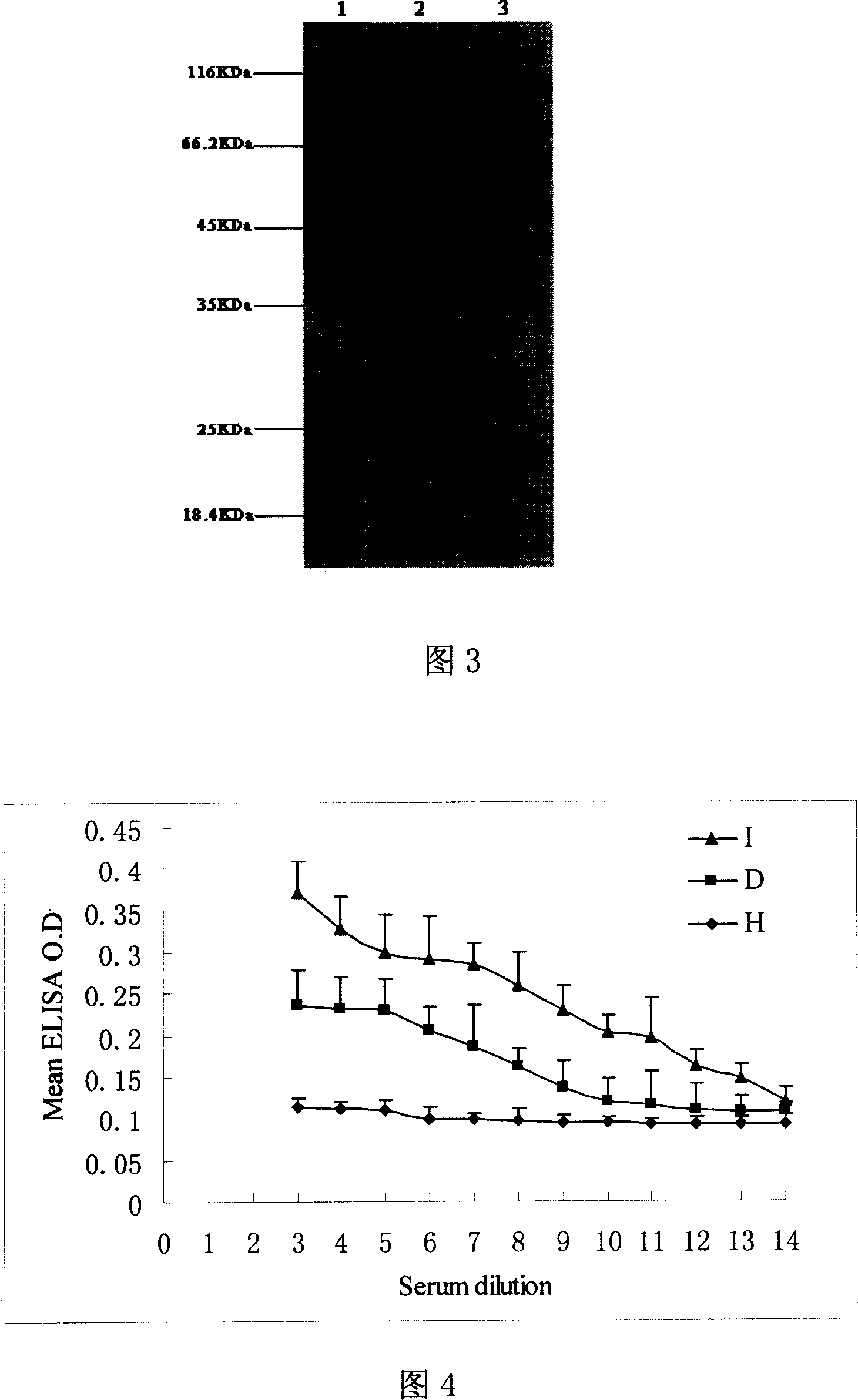
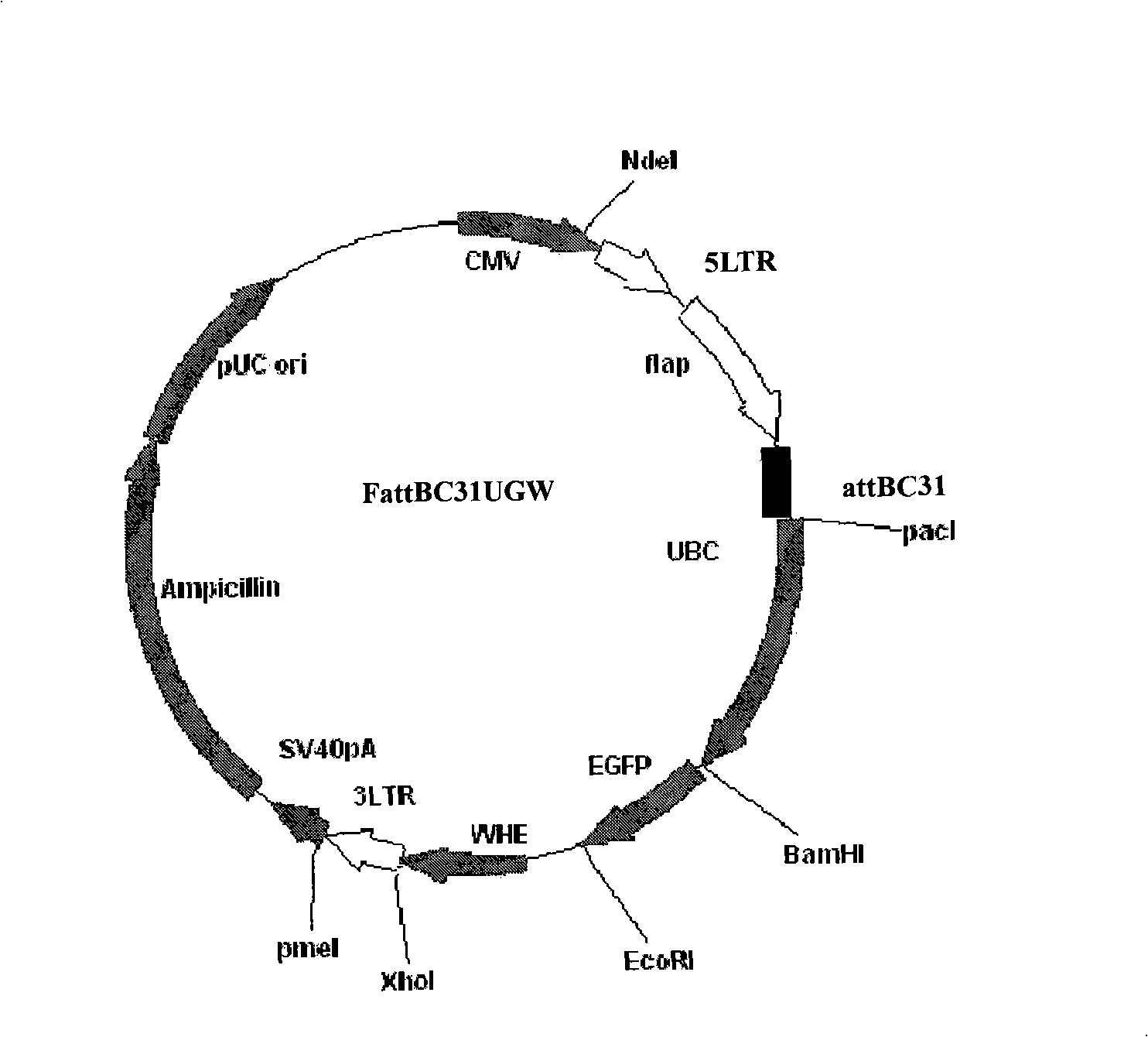
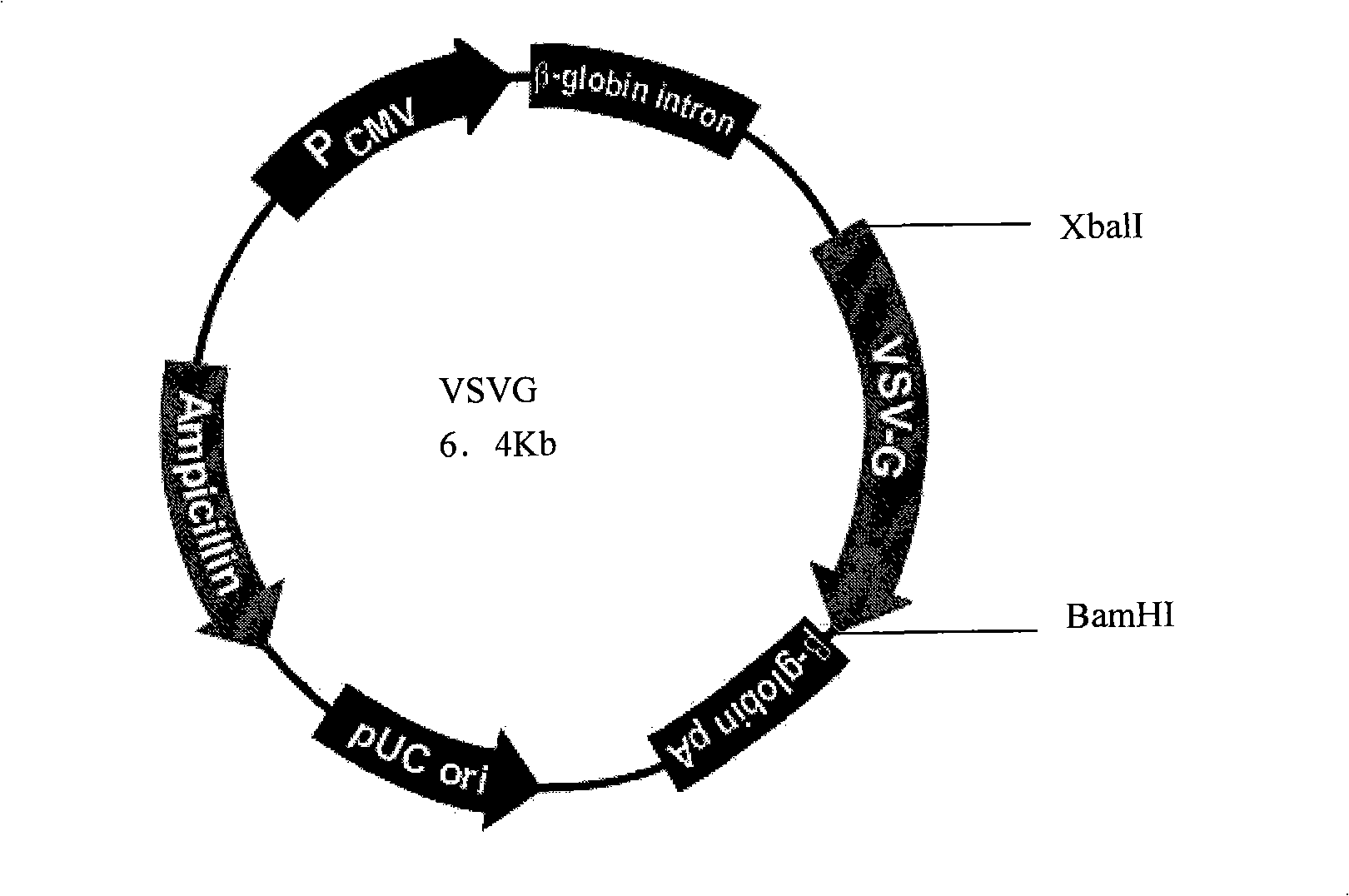
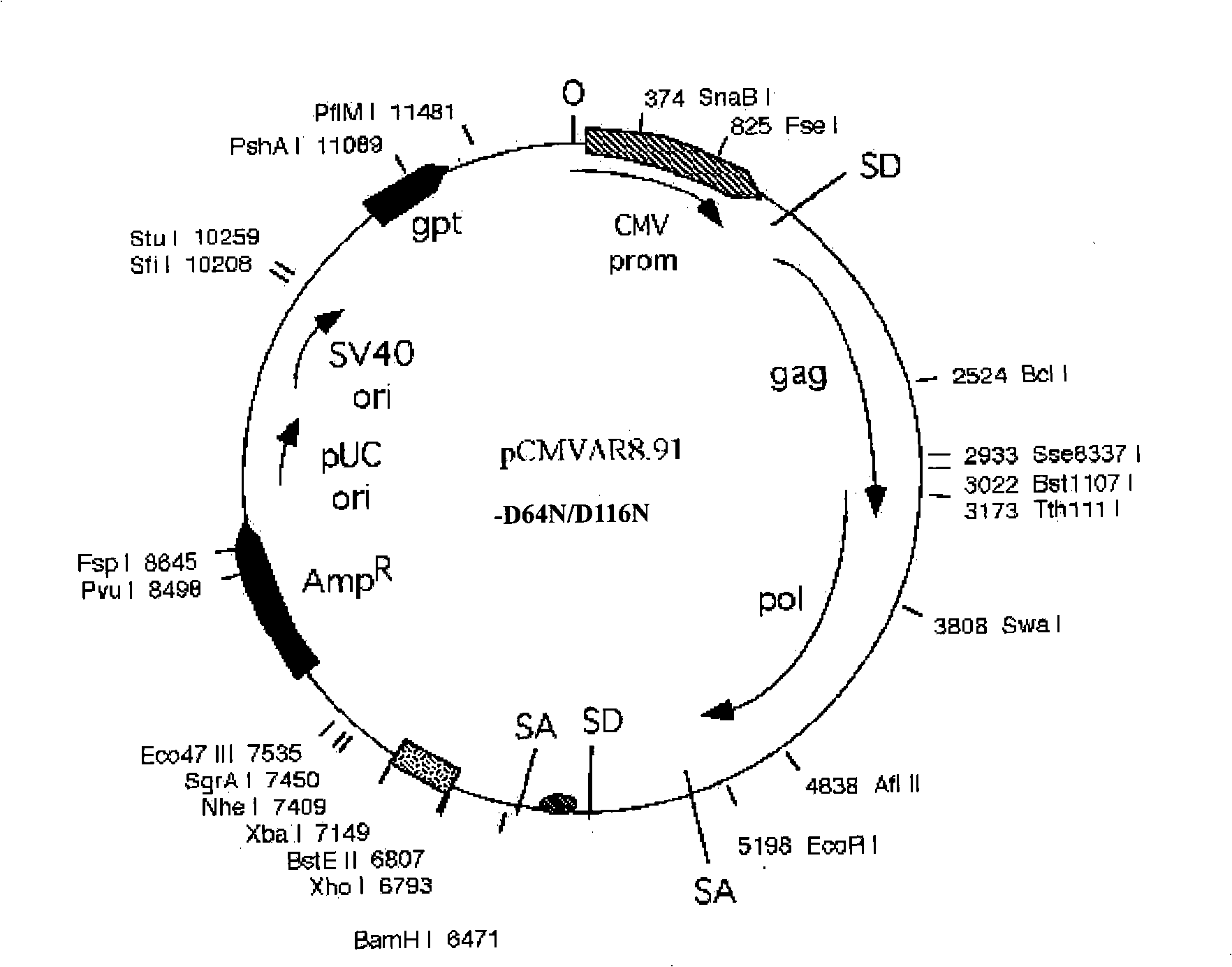
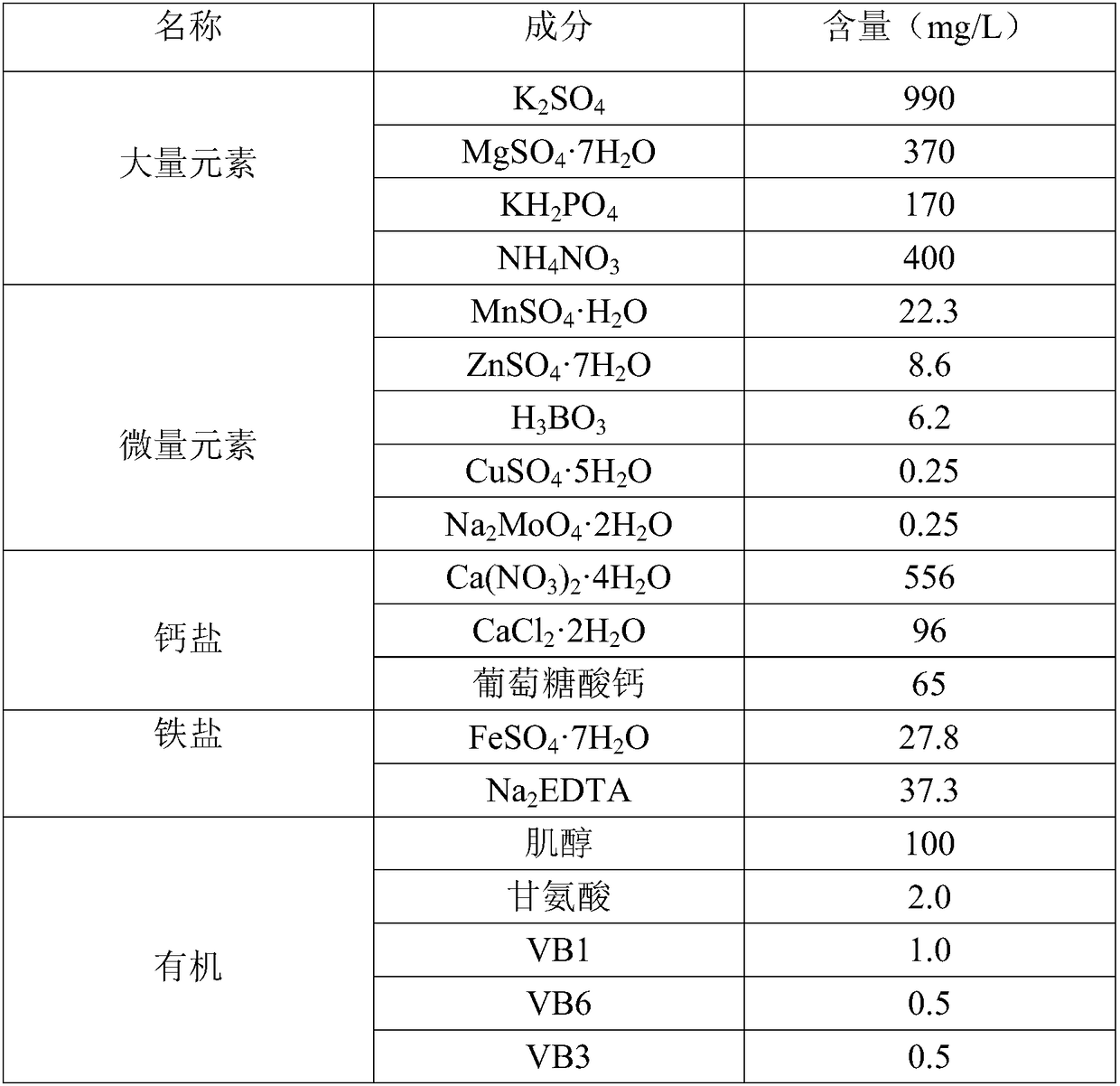
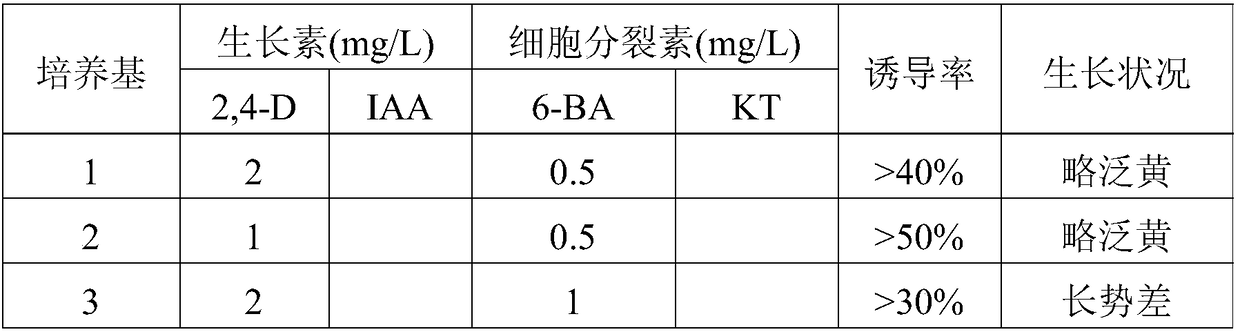
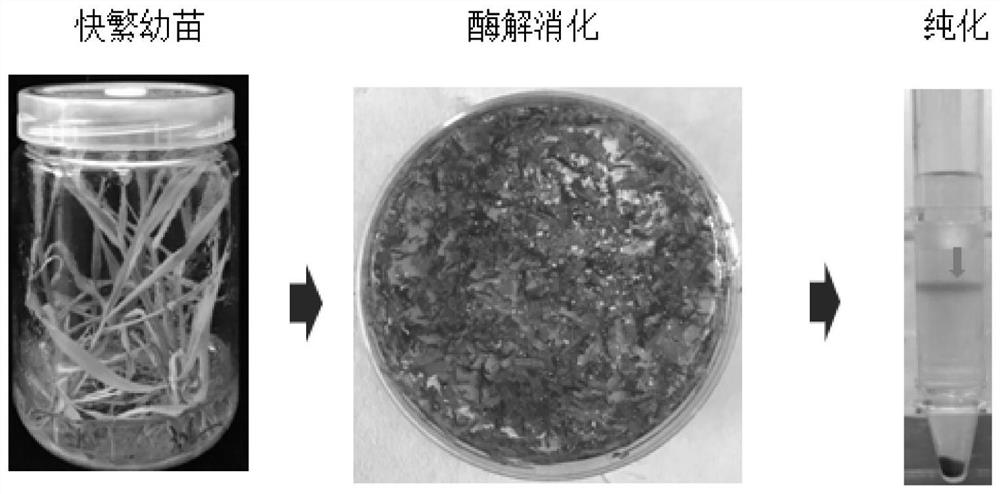
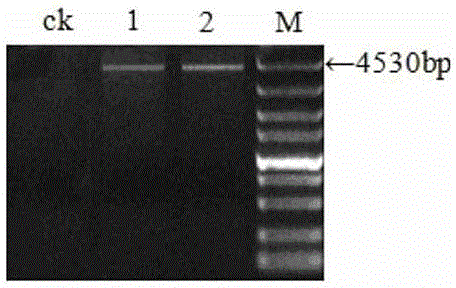
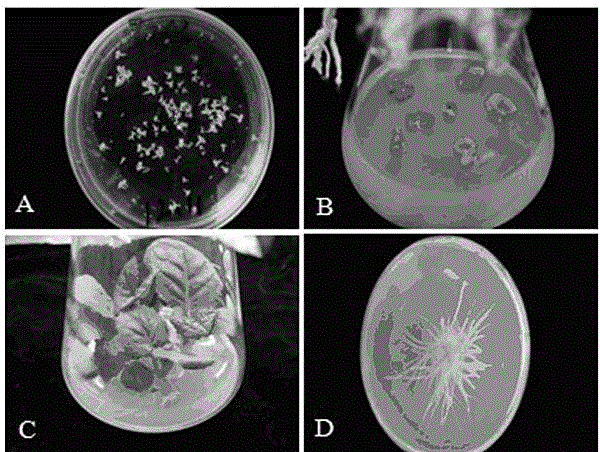
Patents
Literature
236 results about "Cellular engineering" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cellular engineering, principally the control and regulation of cell proliferation, differentiation, and function, is vital to the success of cell-based therapeutic applications and technologies.
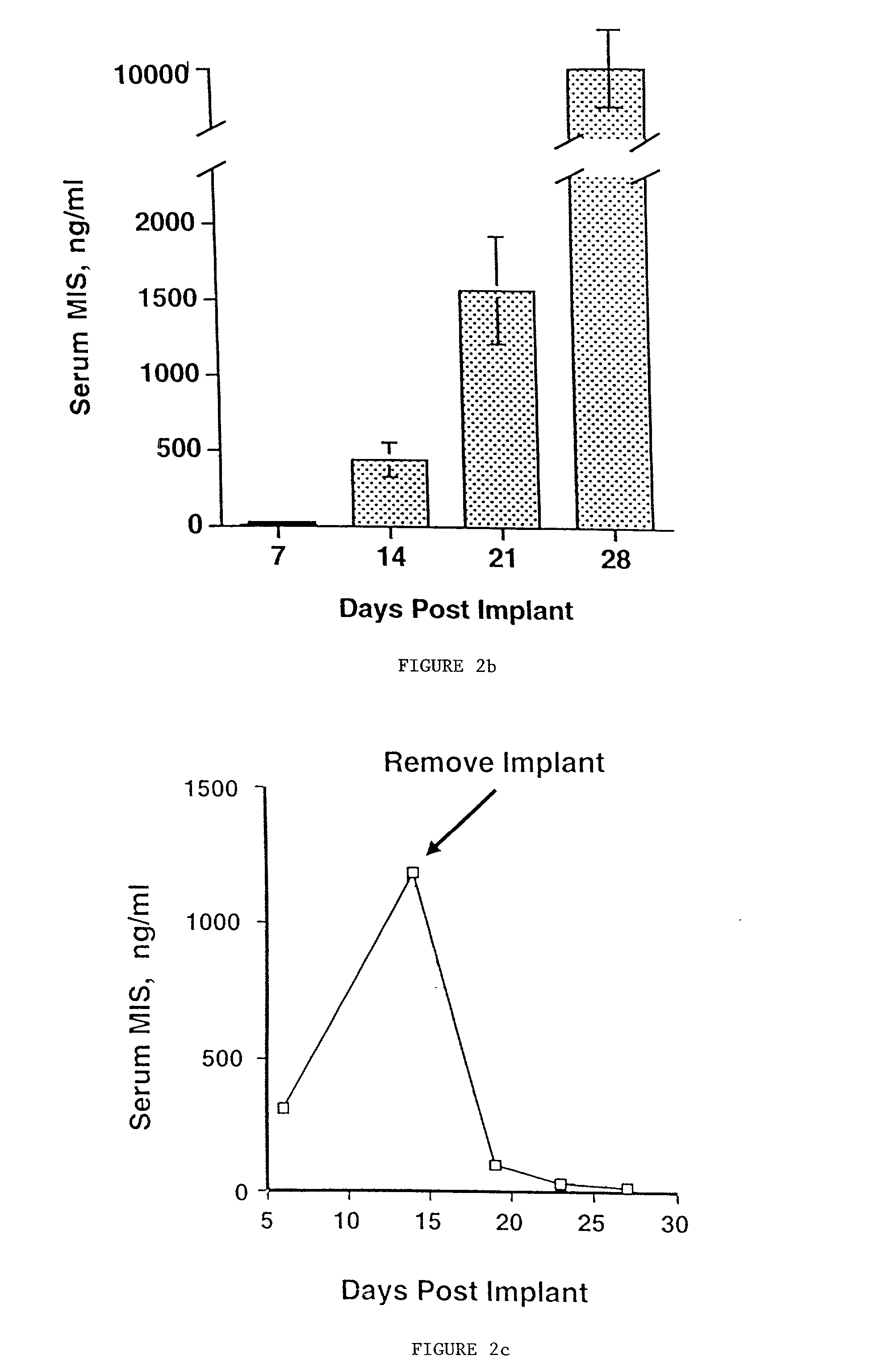
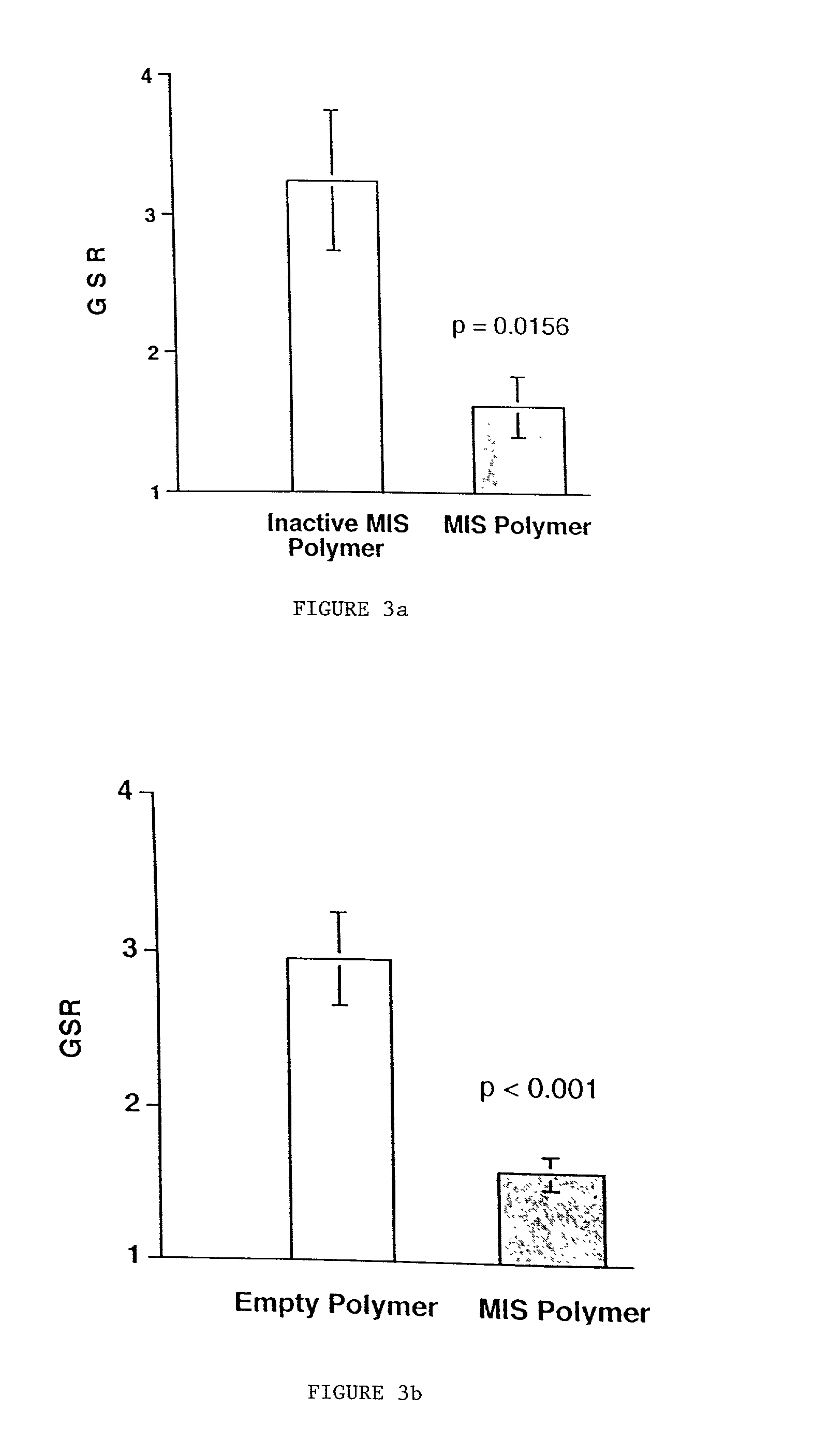
Delivery of therapeutic biologicals from implantable tissue matrices
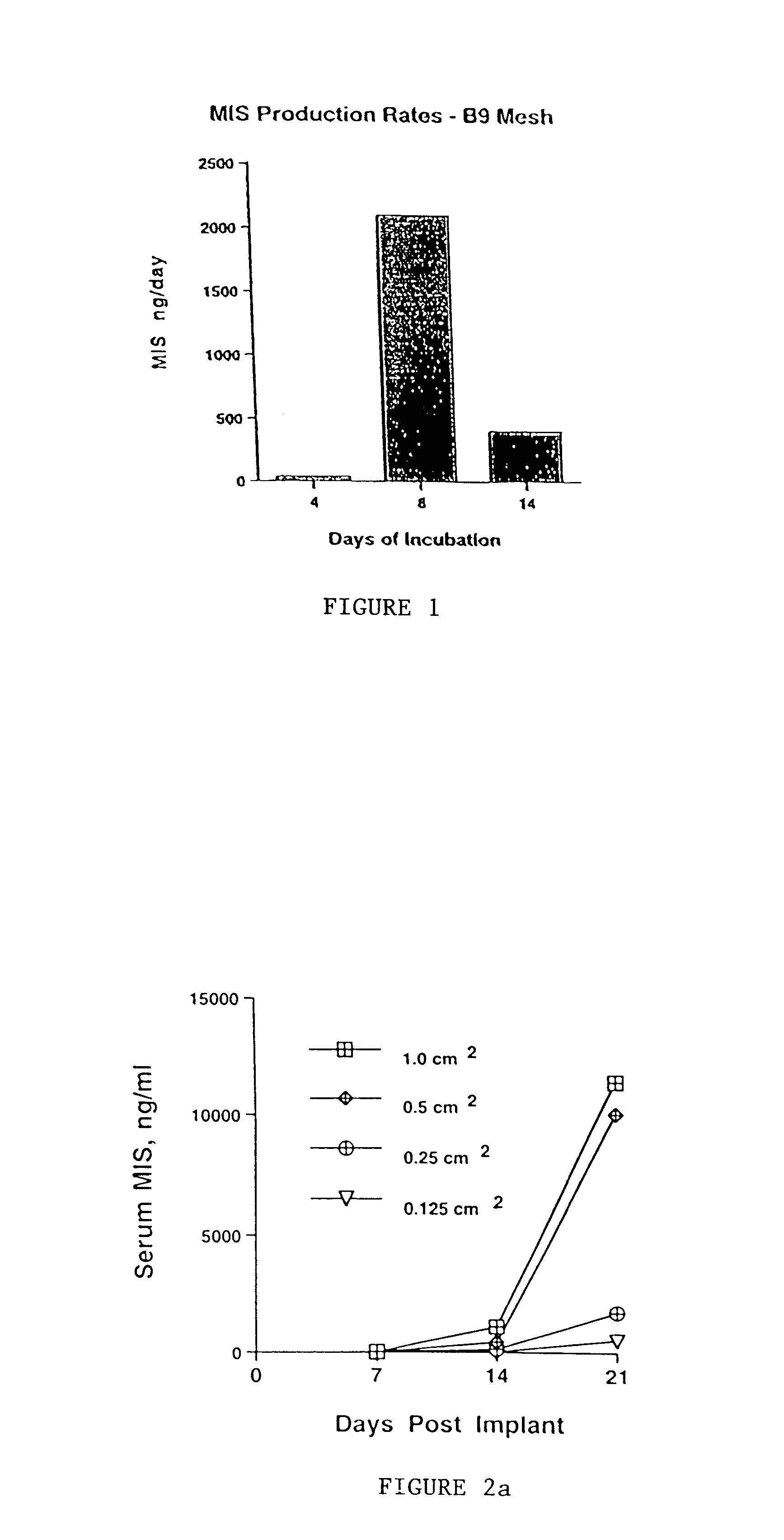
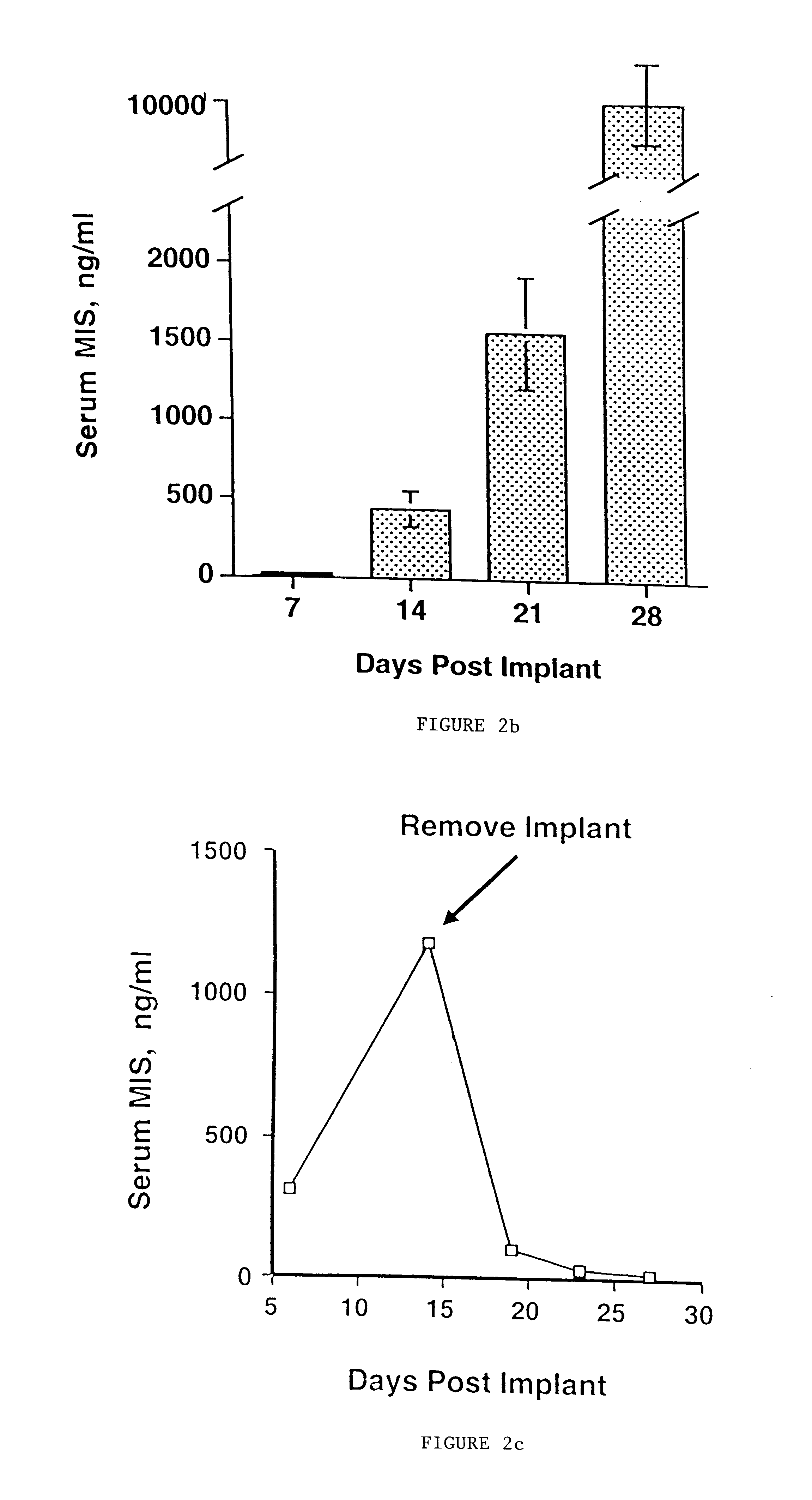
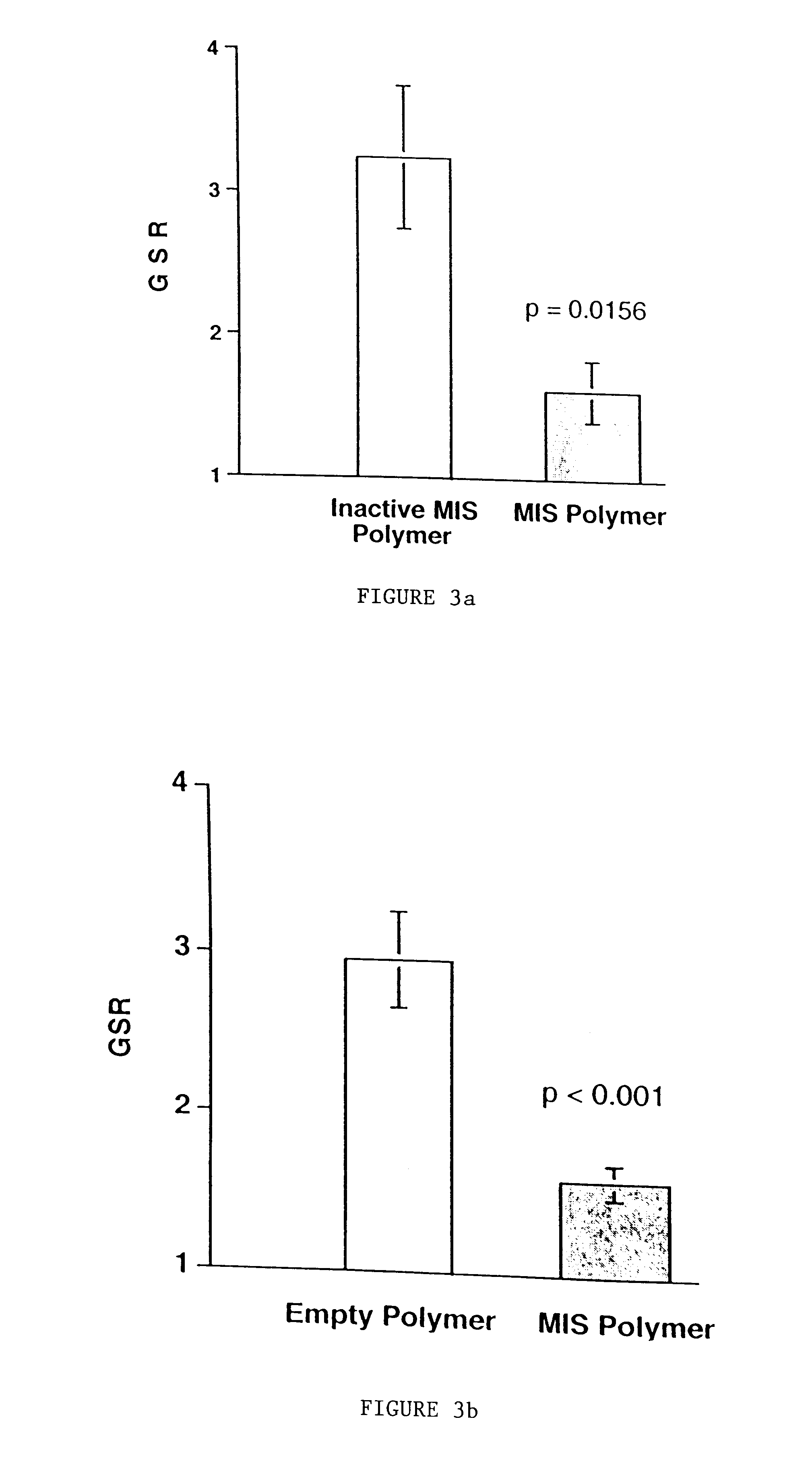
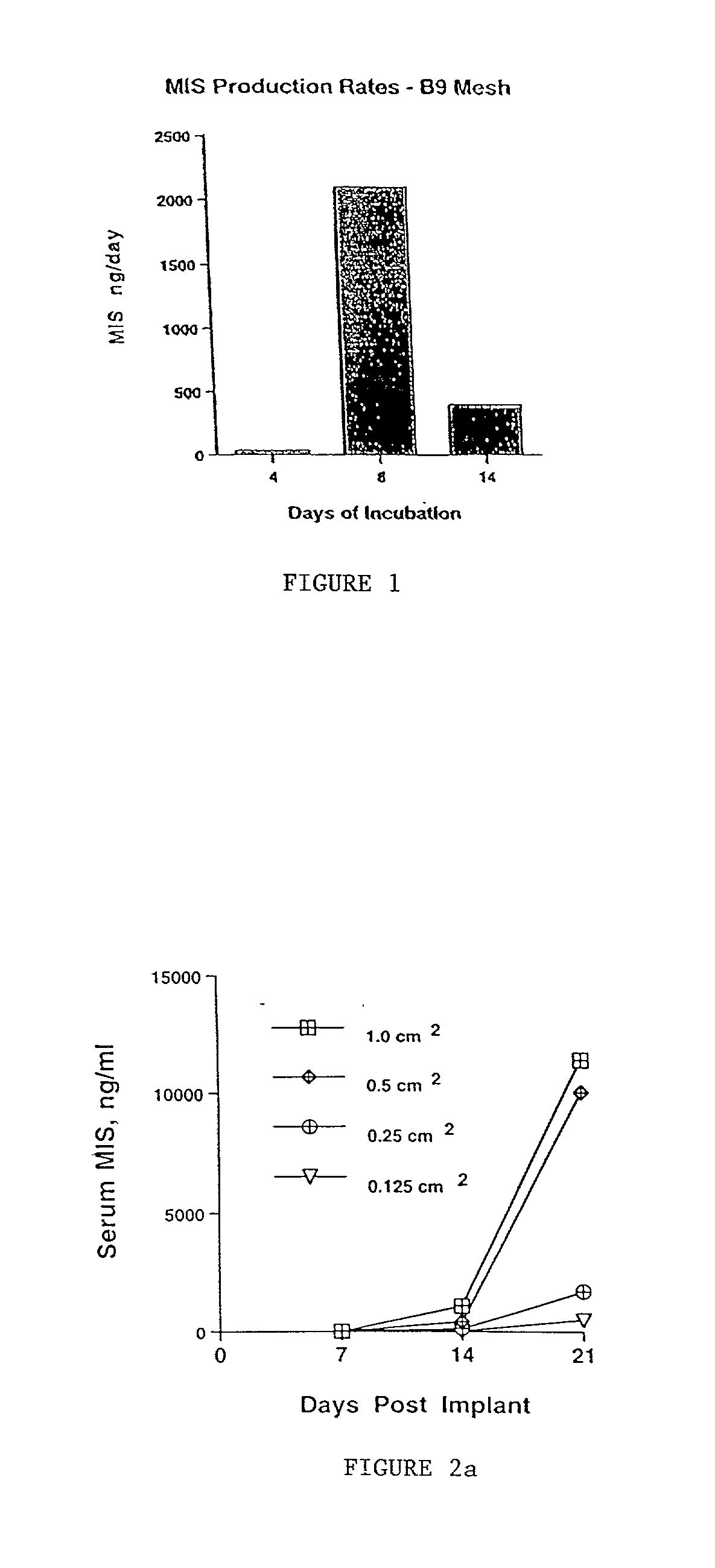
Normal cells, such as fibroblasts or other tissue or organ cell types, are genetically engineered to express biologically active, therapeutic agents, such as proteins that are normally produced in small amounts, for example, MIS, or other members of the TGF-beta family Herceptin(TM), interferons, andanti-angiogenic factors. These cells are seeded into a matrix for implantation into the patient to be treated. Cells may also be engineered to include a lethal gene, so that implanted cells can be destroyed once treatment is completed. Cells can be implanted in a variety of different matrices. In a preferred embodiment, these matrices are implantable and biodegradable over a period of time equal to or less than the expected period of treatment, when cells engraft to form a functional tissue producing the desired biologically active agent. Implantation may be ectopic or in some cases orthotopic. Representative cell types include tissue specific cells, progenitor cells, and stem cells. Matrices can be formed of synthetic or natural materials, by chemical coupling at the time of implantation, using standard techniques for formation of fibrous matrices from polymeric fibers, and using micromachining or microfabrication techniques. These devices and strategies are used as delivery systems via standard or minimally invasive implantation techniques for any number of parenterally deliverable recombinant proteins, particularly those that are difficult to produce in large amounts and / or active forms using conventional methods of purification, for the treatment of a variety of conditions that produce abnormal growth, including treatment of malignant and benign neoplasias, vascular malformations (hemangiomas), inflammatory conditions, keloid formation, abdominal or plural adhesions, endometriosis, congenital or endocrine abnormalities, and other conditions that can produce abnormal growth such as infection. Efficacy of treatment with the therapeutic biologicals is detected by determining specific criteria, for example, cessation of cell proliferation, regression of abnormal tissue, or cell death, or expression of genes or proteins reflecting the above.
Owner:THE GENERAL HOSPITAL CORP
Delivery of therapeutic biologicals from implantable tissue matrices
InactiveUS20020031500A1Many of effectMany of inconveniencePowder deliveryBiocideProgenitorActive agent
Normal cells, such as fibroblasts or other tissue or organ cell types, are genetically engineered to express biologically active, therapeutic agents, such as proteins that are normally produced in small amounts, for example, MIS, or other members of the TGF-beta family Herceptin(TM), interferons, andanti-angiogenic factors. These cells are seeded into a matrix for implantation into the patient to be treated. Cells may also be engineered to include a lethal gene, so that implanted cells can be destroyed once treatment is completed. Cells can be implanted in a variety of different matrices. In a preferred embodiment, these matrices are implantable and biodegradable over a period of time equal to or less than the expected period of treatment, when cells engraft to form a functional tissue producing the desired biologically active agent. Implantation may be ectopic or in some cases orthotopic. Representative cell types include tissue specific cells, progenitor cells, and stem cells. Matrices can be formed of synthetic or natural materials, by chemical coupling at the time of implantation, using standard techniques for formation of fibrous matrices from polymeric fibers, and using micromachining or microfabrication techniques. These devices and strategies are used as delivery systems via standard or minimally invasive implantation techniques for any number of parenterally deliverable recombinant proteins, particularly those that are difficult to produce in large amounts and / or active forms using conventional methods of purification, for the treatment of a variety of conditions that produce abnormal growth, including treatment of malignant and benign neoplasias, vascular malformations (hemangiomas), inflammatory conditions, keloid formation, abdominal or plural adhesions, endometriosis, congenital or endocrine abnormalities, and other conditions that can produce abnormal growth such as infection. Efficacy of treatment with the therapeutic biologicals is detected by determining specific criteria, for example, cessation of cell proliferation, regression of abnormal tissue, or cell death, or expression of genes or proteins reflecting the above.
Owner:THE GENERAL HOSPITAL CORP
Tissue culture and rapid propagation method for pinellia tuber plant
InactiveCN102150624AReduce the numberIncrease the level of automationPlant tissue cultureHorticulture methodsMedicinal herbsPlant cell
The invention relates to a tissue culture and rapid propagation method for a pinellia tuber plant and belongs to the technical field of plant cell engineering. Organs such as sterile blades, leaf stalks, cluster buds and the like of pinellia tuber are used as inoculating materials. The method comprises the following steps of: transplanting the inoculating materials into an intermittent submerged culture reactor, and performing two stages of proliferating and inducing, and rooting and generating and culturing tubers; and after the proliferating culture is finished, replacing a proliferation culture medium with a rooting and tuber generating culture medium under the aseptic condition so as to promote the forming of the tubers of the pinellia tuber. By the method, the automation degree in the production process of pinellia tuber seedlings is greatly improved, the human input is reduced during the culture, and the proliferation rate is greatly improved. The pinellia tuber seedlings which are produced by the reactor have the advantages of no pathogenic bacteria, uniform and stable heredity and the like. The survival rate is obviously improved, the labor cost is obviously lowered, and the guarantee for producing a great number of high-quality seedlings and isolated tubers at low cost is provided. By the method, the seedling quality during the plantation of pinellia tuber medicinal materials is improved, the seedling cost is lowered, and economic benefits are obviously improved and the same time.
Owner:NANJING UNIV OF TECH
Method for improving de-cellular system engineering valve/blood vessel stent
The invention discloses a method for linking a RGD improved acellular tissue engineering valve / intravascular stent with epoxy chloropropane. The method, by using epoxy groups of the epoxy chloropropane, cross links and fixes the functional domain RGD (YGRGDSP) polypeptide of cell adhesion and recognition receptors on an acellular tissue engineering valve / intravascular stent material; meanwhile, the chemical crosslinking function of the epoxy chloropropane can fully cross link the collagen protein which is the main component of the acellular stent material so as to achieve the purposes of anti-calcification and reducing the zymolysis speed inside the stent material, and finally proving the improved acellular tissue engineering valve / intravascular stent with better biological and mechanical properties, higher adhesion rate toward seed cell, and better property of delaying enzyme digestion and anti-calcification, therefore, the acellular tissue engineering valve / intravascular stent can actually meet the requirements of tissue engineering valves.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Serum-free protein-free culture medium supporting HEK293 cell suspension culture and preparation method and application thereof
ActiveCN111304149AEasy to separate and purifyReduce pollutionCulture processArtificial cell constructsBiotechnologyFree protein
The invention discloses a serum-free protein-free culture medium supporting HEK293 cell suspension culture and a preparation method and application thereof, and belongs to the technical field of cellengineering. According to the serum-free protein-free culture medium disclosed by the invention, different nutrient substances are selected according to the nutritional requirements of in-vitro growthof cells so as to replace animal serum, and the proportion of the different nutrient substances is reasonably adjusted. By further adding an anti-shearing force substance and an anti-caking substance, high-density suspension culture of HEK293 cells can be maintained without supplementing serum, and the normal form of the cells is maintained, so that the cells are dispersed and not caked, meanwhile, the normal growth speed is maintained, and the transfection and expression of target genes are facilitated.
Owner:XINXIANG MEDICAL UNIV +1
Monoclonal antibody of immunoglobulin of anti lymphocyst vitos of Pacific fluke, and preparation method
ActiveCN101003572AInnovative designAchieve purificationImmunoglobulins against animals/humansBiological testingBALB/cCell engineering
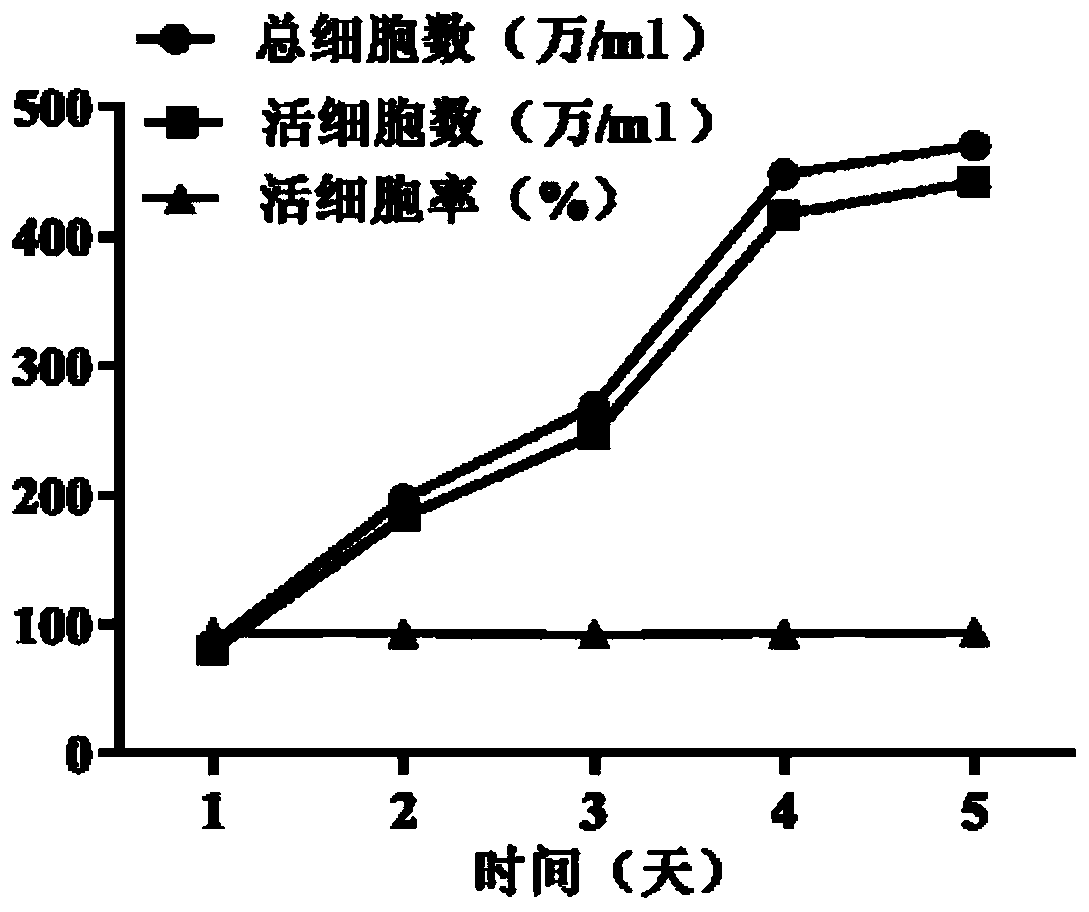
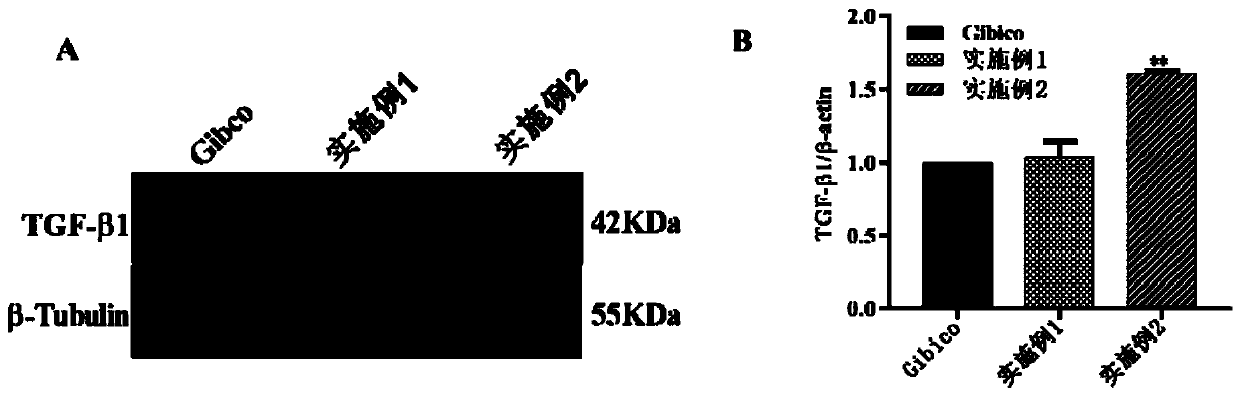
This invention discloses monoclonal antibody against anti-LCDV immunoglobulin of Paralichthys olivaceus, which is excreted by hybridoma JF-lgM-H (CCTCC-C200631). The method comprises: immunizing Paralichthys olivaceus with LCDV inactivated by formalin to prepare antiserum, purifying Paralichthys olivaceus immunoglobulin, immunizing Balb / c mice as antigen, preparing hybridoma cells by cell engineering method, and screening the monoclonal antibody by immunoassay. Indirect ELISA and indirect immunofluorescent antibody assay show that this monoclonal antibody is located on the heavy chain (7-80 kDa) of the anti-LCDV immunoglobulin. The monoclonal antibody can be used for preparing reagents for detecting LCDV infection in early stage, and evaluating the immune effects of LCDV vaccine inactivated by formalin.
Owner:OCEAN UNIV OF CHINA
Site-specific integration retroviral vector system and preparation thereof
InactiveCN101302537AAvoid toxicityOvercoming the Risk of Insertion MutationsGenetic engineeringFermentationTransgeneViral vector
Owner:北京康爱瑞浩细胞技术有限公司
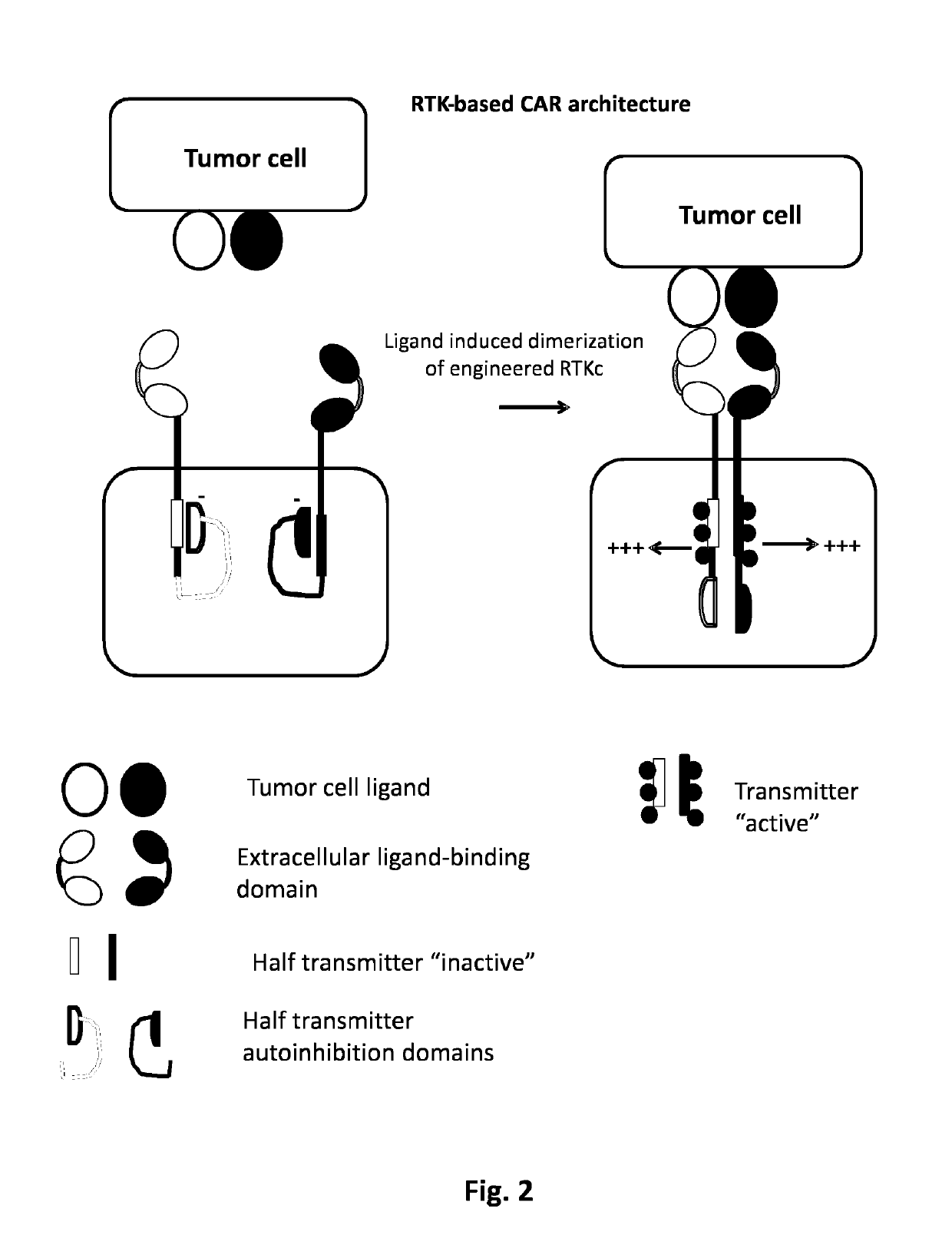
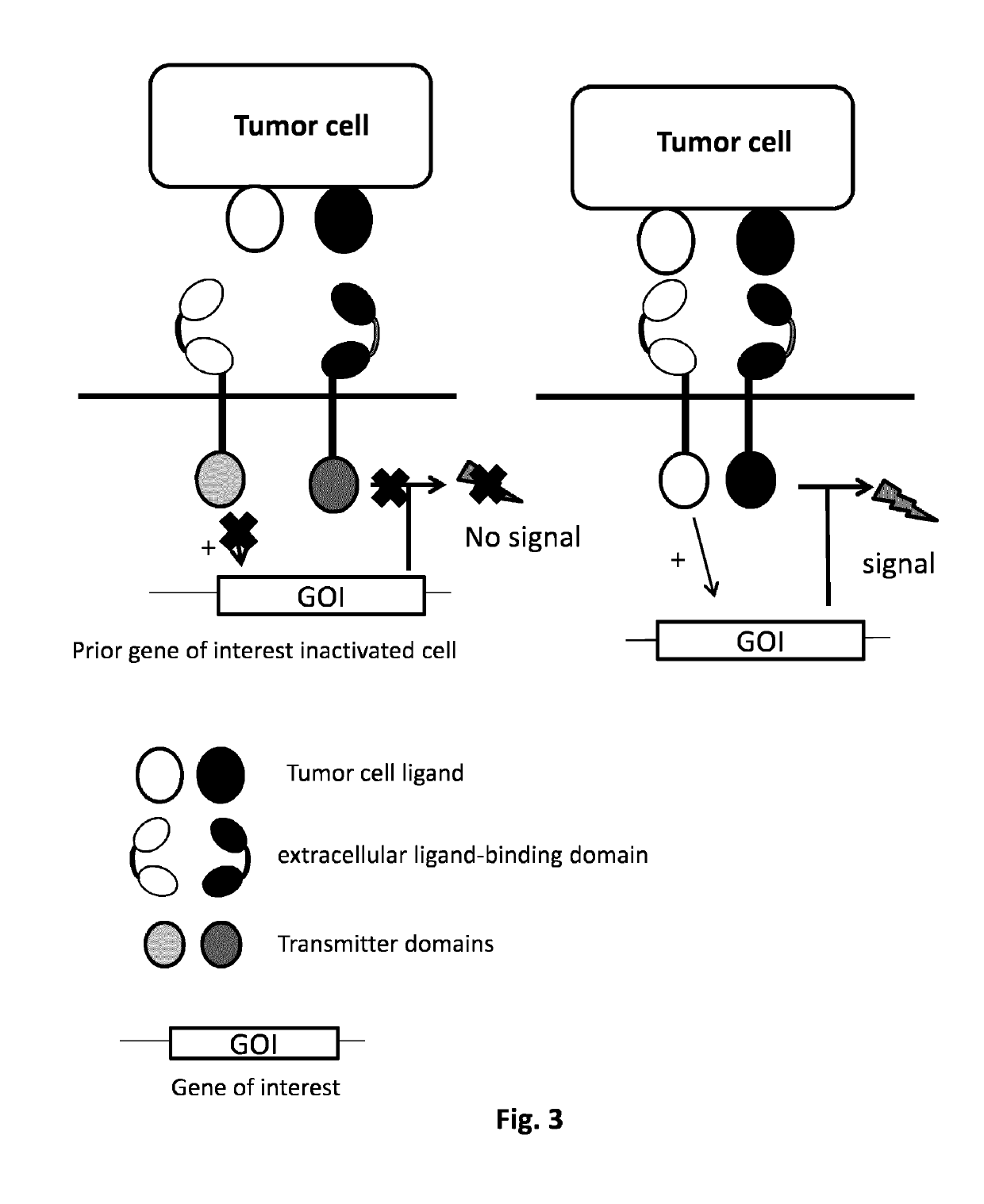
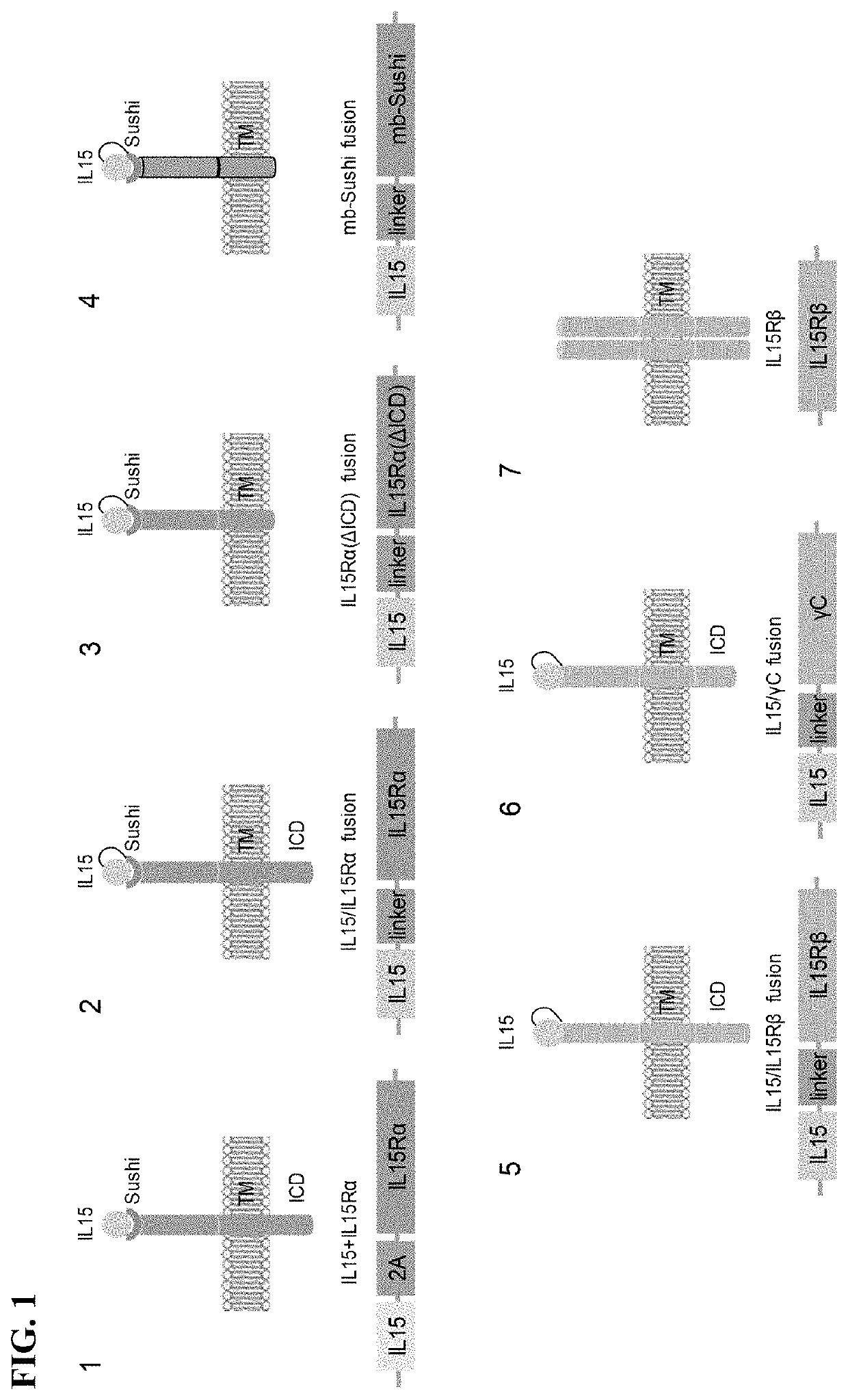
Method of engineering multi-input signal sensitive T cell for immunotherapy
ActiveUS10239948B2Polypeptide with localisation/targeting motifHybrid immunoglobulinsAntigen receptorsBinding domain
The present invention relates to a method to engineer immune cell for immunotherapy. In particular said immune cells are engineered with chimeric antigen receptors, which be activated by the combination of hypoxia and ligand extracellular binding as input signals. The invention also relates to new designed chimeric antigen receptors which are able to redirect immune cell specificity and reactivity toward a selected target exploiting the ligand-binding domain properties and the hypoxia condition. The present invention also relates to cells obtained by the present method, in particular T-cells, comprising said chimeric antigen receptors for use in cancer treatments.
Owner:CELLECTIS SA
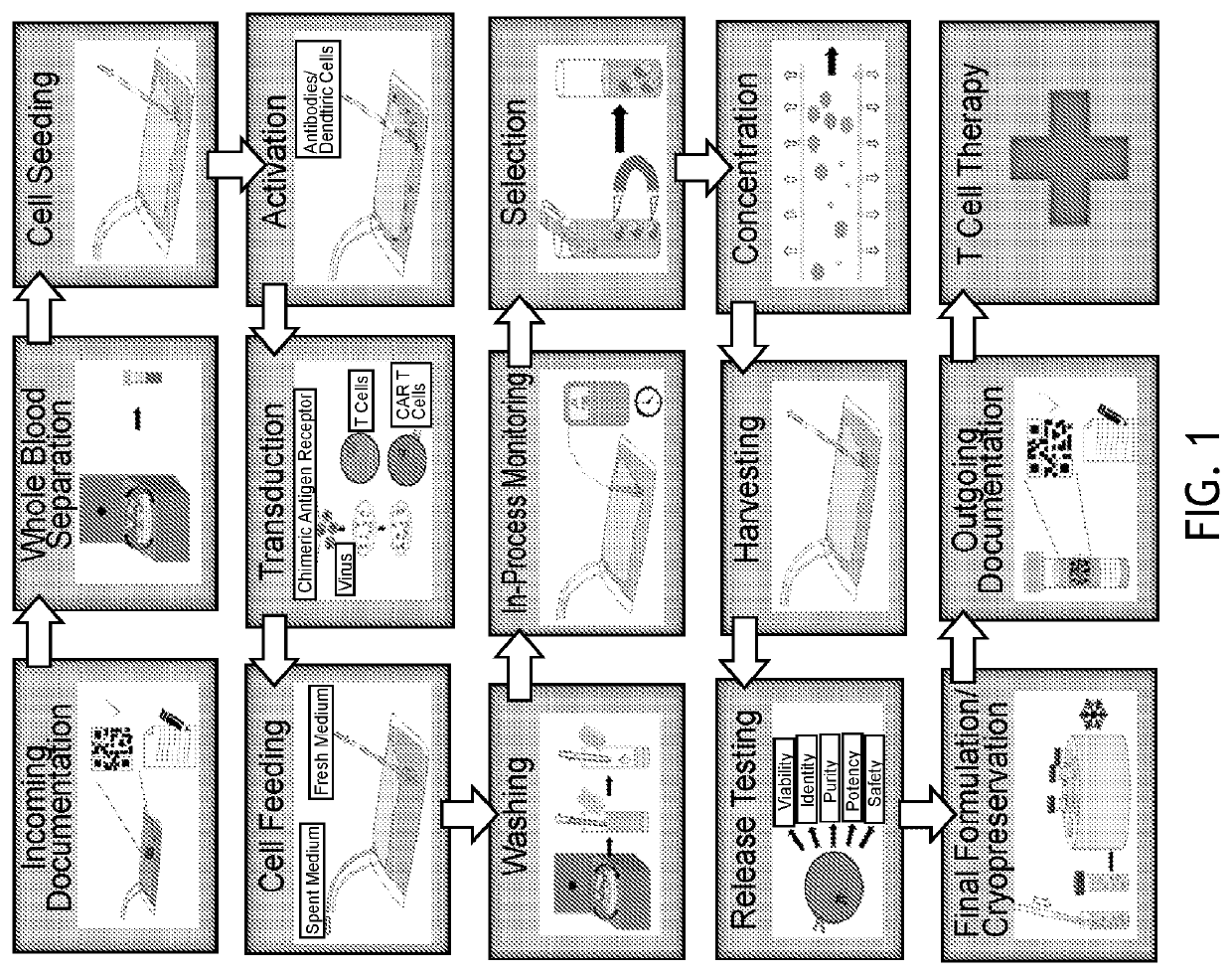
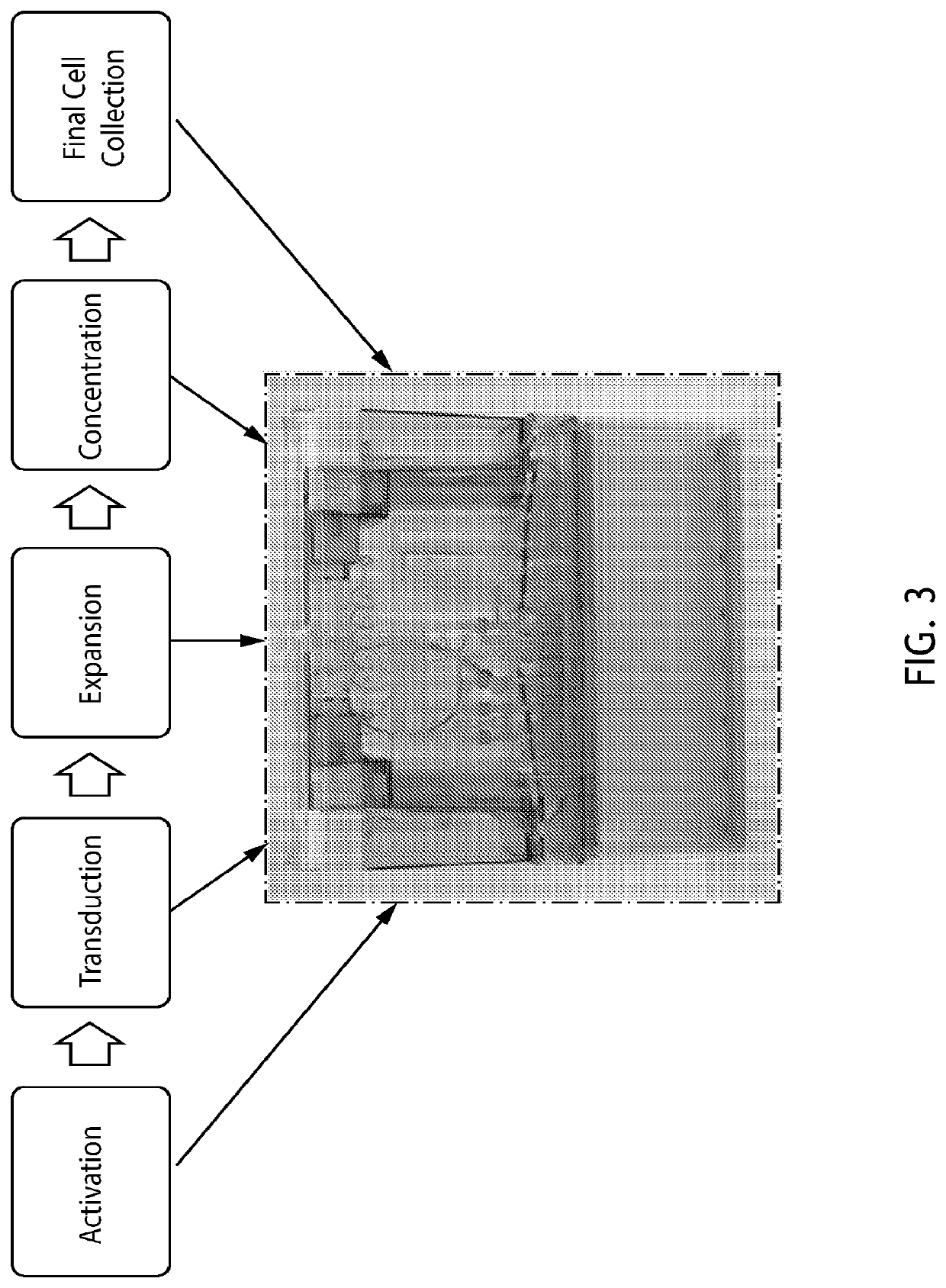
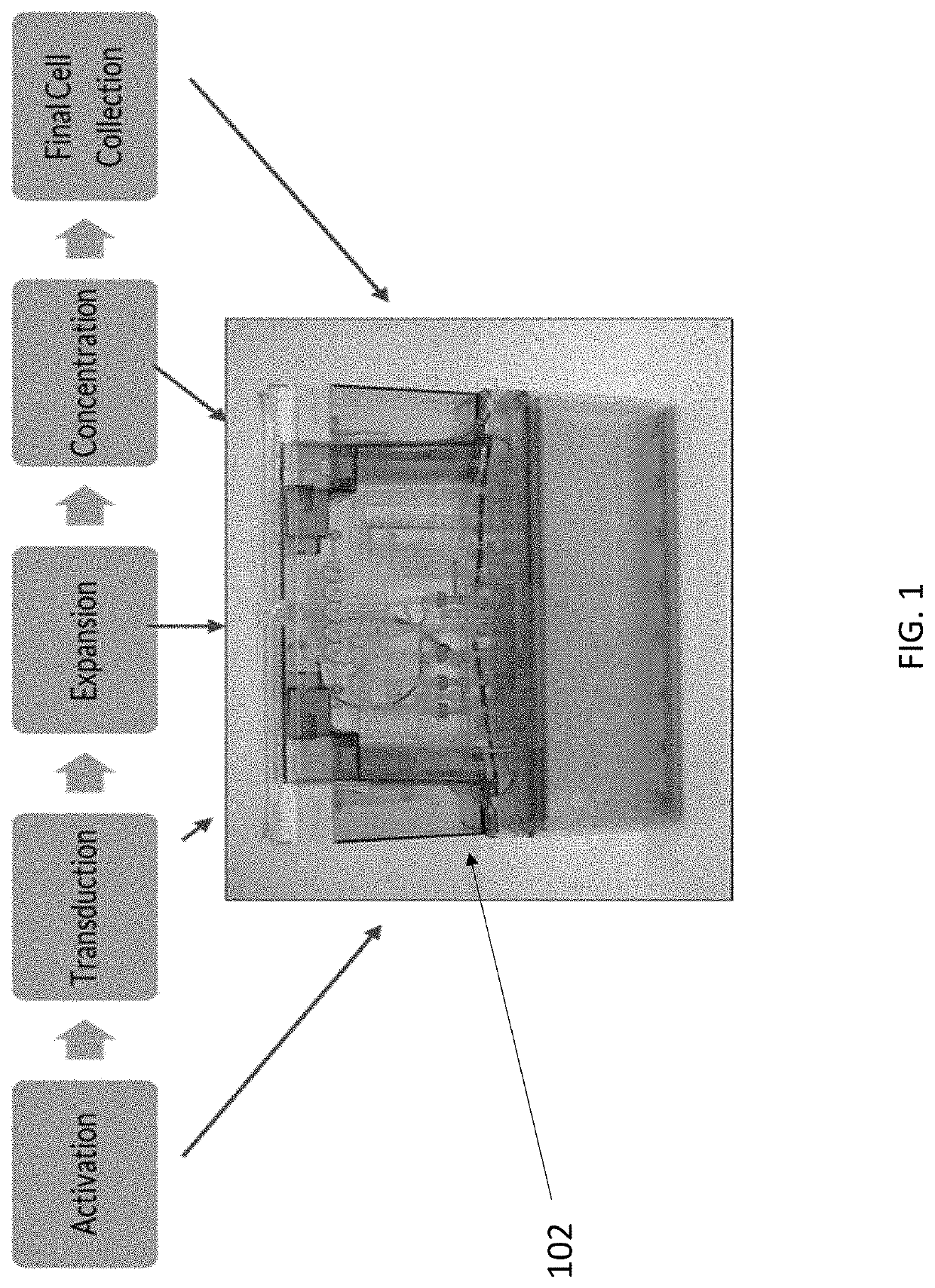
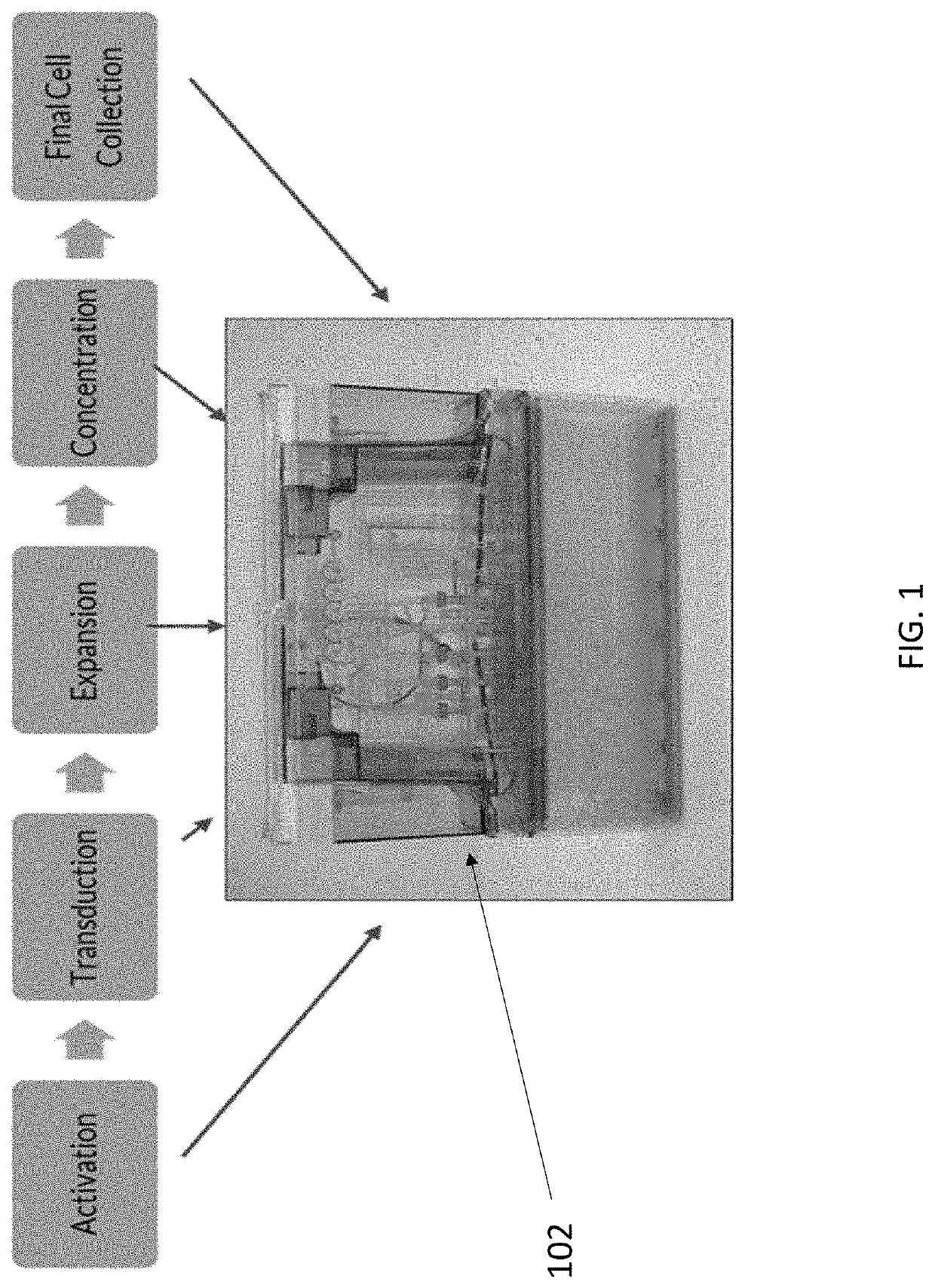
End-to-end cell therapy automation
ActiveUS20200071670A1Increase workloadBioreactor/fermenter combinationsBiological substance pretreatmentsAntigen receptorCell therapy
The present disclosure provides an automated method of producing genetically modified immune cells, including chimeric antigen receptor T (CAR T) cells, utilizing a fully-enclosed cell engineering system.
Owner:OCTANE BIOTECH +2
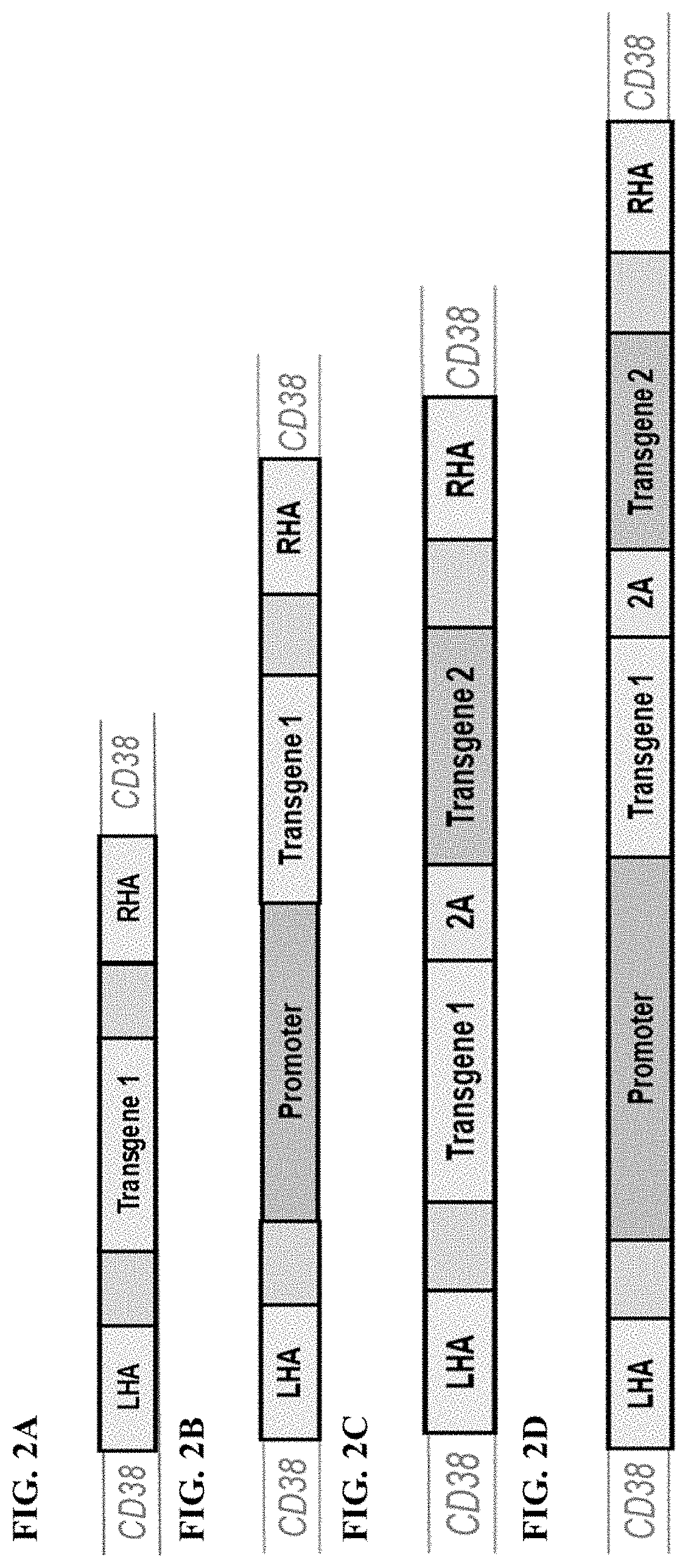
Genetic transformation method for acquiring Taxus chinensis transgenic callus
InactiveCN102690841ASolve the shortage of medicine resourcesGenetic engineeringFermentationGene engineeringCell engineering
The invention belongs to the technical field of biology, and particularly relates to a genetic transformation method for acquiring Taxus chinensis transgenic callus, which comprises the following steps: carrying out induced culture on Taxus chinensis embryo callus, transferring efficient expression vector carrying GUS gene into root-cancer agrobacterium, genetically transforming the embryo callus with the agrobacterium, carrying out enrichment subculture on the transformation receptor material, screening the resistant embryo callus, carrying out enrichment subculture on the resistant embryo callus, carrying out PCR (polymerase chain reaction) detection, detecting the GUS gene, and the like. According to the genetic transformation method, the GUS gene is expressed in the Taxus chinensis embryo callus to successfully acquire the Taxus chinensis embryo callus. The Taxus chinensis transgenic callus acquired by the method provided by the invention can be widely used in Taxus chinensis gene engineering, cell engineering, metabolism engineering and establishment of a Taxus chinensis genetic transformation system.
Owner:FUDAN UNIV
GPC3 (glypican-3) monoclonal antibody hybridoma strain 8G6, and preparation method and application thereof
ActiveCN102634487AReduce manufacturing costFunction increaseImmunoglobulins against animals/humansTissue cultureMicrobiological cultureMonoclonal
The invention discloses a GPC3 (glypican-3) monoclonal antibody hybridoma strain 8G6, and a preparation method and application thereof, which belong to the technical field of cell engineering. The preservation code of the GPC3 monoclonal antibody hybridoma strain 8G6 is CGMCC (china general microbiological culture collection center) No.5427. The preparation method of the GPC3 monoclonal antibody hybridoma strain 8G6 includes: using GPC3 protein as immunogen to immunize a mouse; and fusing mouse splenocytes having serum titer more than 1:104 with SP2 / 0 myeloma cells, using an HATRPMI-1640 medium to screen fusion cells, and finally obtaining the hybridoma strain 8G6 by ELISA (enzyme-linked immunosorbent assay) and repeated limiting dilution. The GPC3 monoclonal antibody hybridoma strain 8G6 is high in yield of secretory antibodies, and is easy to survive, and the secreted monoclonal antibodies are high in titer, sensitive in reaction, easy to detect, low in production cost and widely applicable to detection of GPC3 protein expression.
Owner:GUANGZHOU DARUI BIOTECH
Preparation method of immortalized human cartilage endplate stem cell line and use of immortalized human cartilage endplate stem cell line
InactiveCN103865877AStrong osteogenic differentiation abilityLow priceMicroorganism based processesFermentationBone tissue engineeringStem cell line
The invention belongs to the technical field of cell engineering, and particularly relates to a preparation method of an immortalized human cartilage endplate stem cell line and use of the immortalized human cartilage endplate stem cell line. The invention aims to solve the technical problem of providing an effective preparation method for constructing the immortalized human cartilage endplate stem cell line. The technical scheme is as follows: the preparation method for constructing the immortalized human cartilage endplate stem cell line by adopting the lentiviral transfection technology comprises the following steps: a, constructing a SV40T antigen virus vector; b, carrying out gene transfection of a human endplate stem cell on SV40T antigen; and c, passaging and sieving to obtain the immortalized human cartilage endplate stem cell line. The invention further provides the use of the immortalized human cartilage endplate stem cell line prepared by the method in preparation of bone tissue engineering materials. The immortalized human cartilage endplate stem cell line provided by the invention can be applied to preparation of a tissue-engineered bone and clinically provides a seed cell of the bone tissue engineering low in price and strong in osteogenic capability for the patients with a series of long bone defects and bone nonunion.
Owner:THE SECOND AFFILIATED HOSPITAL ARMY MEDICAL UNIV
Sphingolipid ceramide N-deacylase, methods for producing sphingolipids and sphingolipid derivatives, and sphingolipid ceramide N-deacylase gene
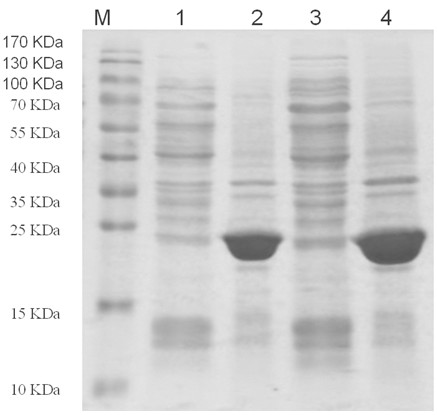
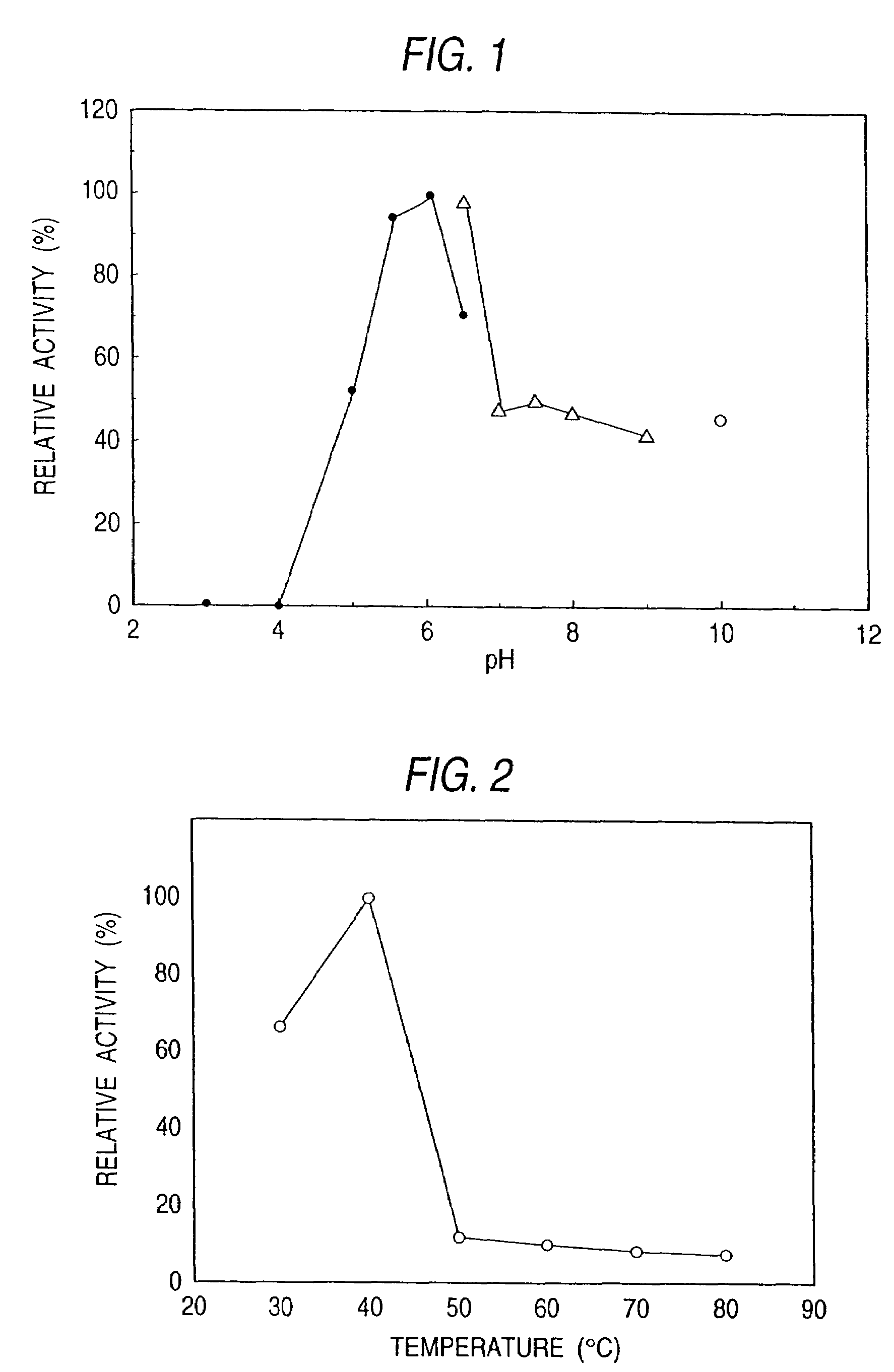
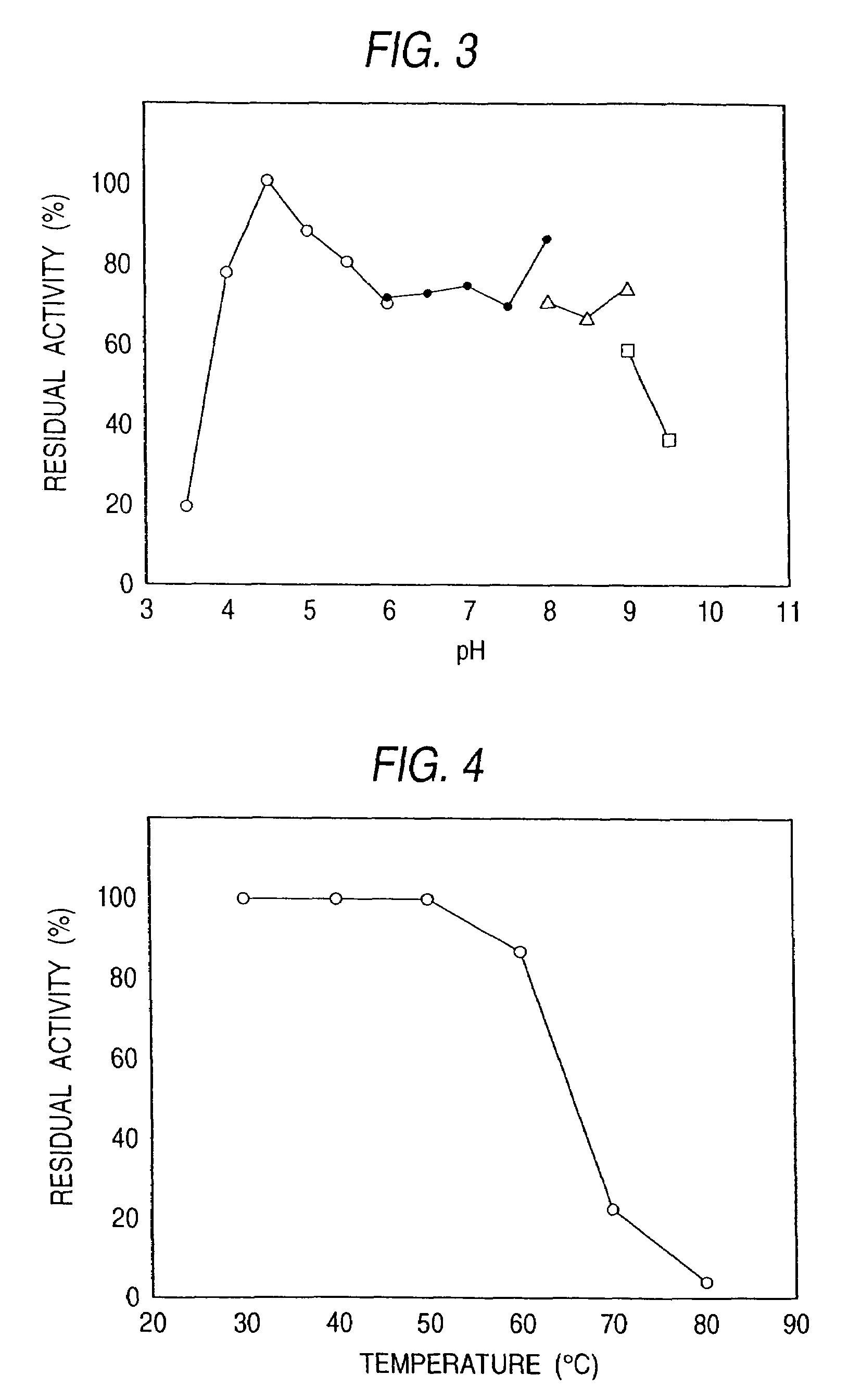
The present invention relates to a novel sphingolipid ceramide N-deacylase (SCDase) having a wide substrate specificity; a method for enzymatically producing a lysosphingolipid or a sphingolipid derivative using the SCDase which is useful in the fields of medicine, carbohydrate engineering, cell engineering, and the like; the lysosphingolipid or sphingolipid derivative obtained by this production method; a gene which encodes a polypeptide having an SCDase activity useful in sphingolipid technology; a method for industrially producing a polypeptide having an SCDase deacylase activity and a recombinant polypeptide thereof using a transformant to which the gene is introduced; a probe or primer which hybridizes to the gene; and an antibody or a fragment thereof which specifically binds to the polypeptide.
Owner:TAKARA HOLDINGS
Culture method for akebiaquinata decne seedlings
InactiveCN104798688AImprove multiplication efficiencyHigh proliferation rateHorticulture methodsPlant tissue cultureBiotechnologyPlant cell
The invention belongs to the technical field of plant cell engineering. Culture steps of akebiaquinata decne seedlings sequentially comprise pretreatment, aseptic explant obtaining, bud tissue culture, callus induced proliferation, embryoid induced proliferation, rooting culture and transplanting. The technical scheme for producing the akebiaquinata decne seedlings, provided by the invention, has the beneficial effects of high proliferation ratio, high biological yield, short culture period, good quality of tissue culture seedlings, healthy seedlings and high cultivation survival rate. Compared with other culture modes, a culture method for culturing the akebiaquinata decne seedlings by using pretreatment, aseptic explant obtaining, bud tissue culture, callus induced proliferation, embryoid induced proliferation, rooting culture and transplanting, disclosed by the invention, has the advantages of high propagation rate, short culture period, convenience in transfer, healthy seedlings, high cultivation survival rate and low cost, and is suitable for scale culture and production of the akebiaquinata decne seedlings.
Owner:JIUJIANG UNIVERSITY +1
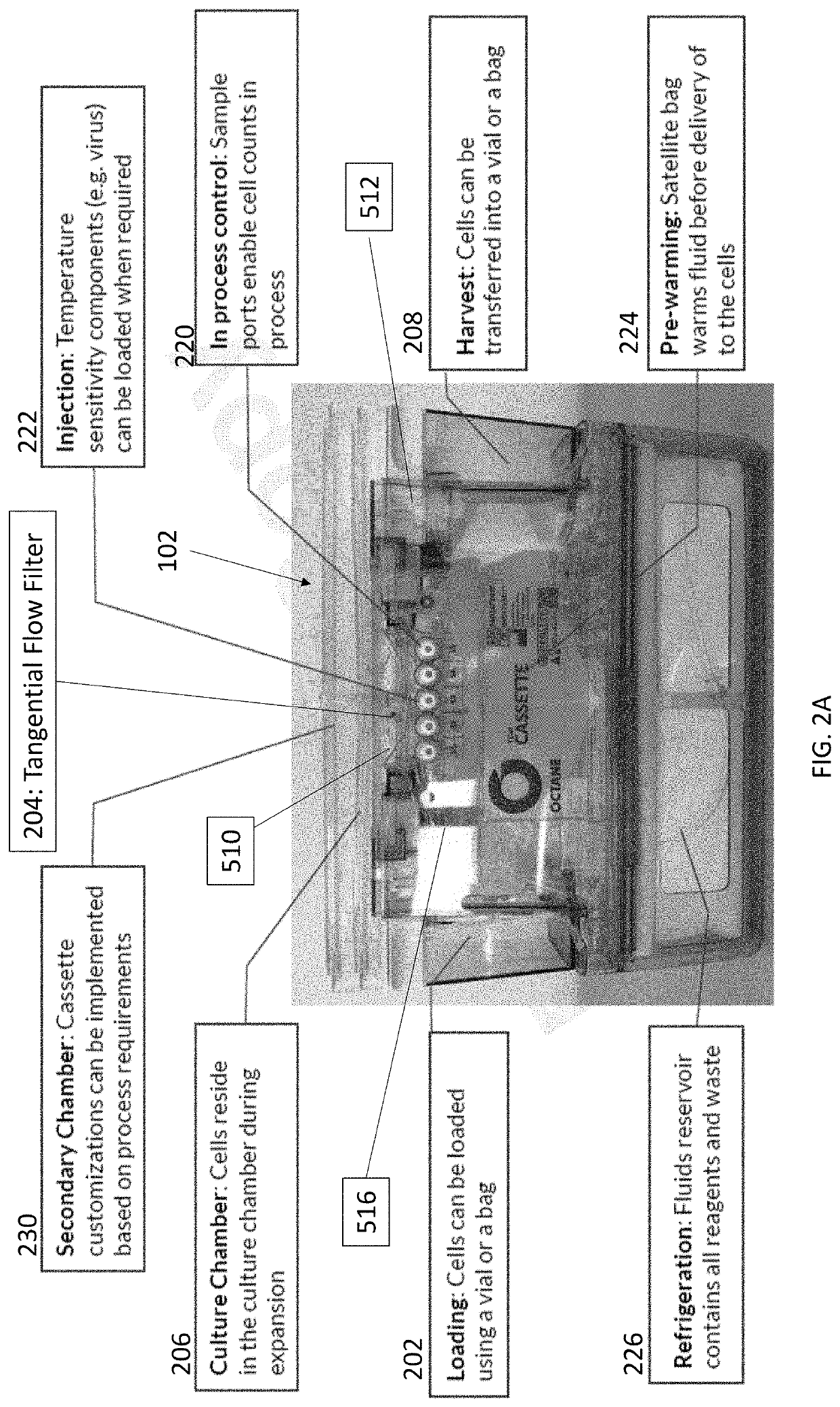
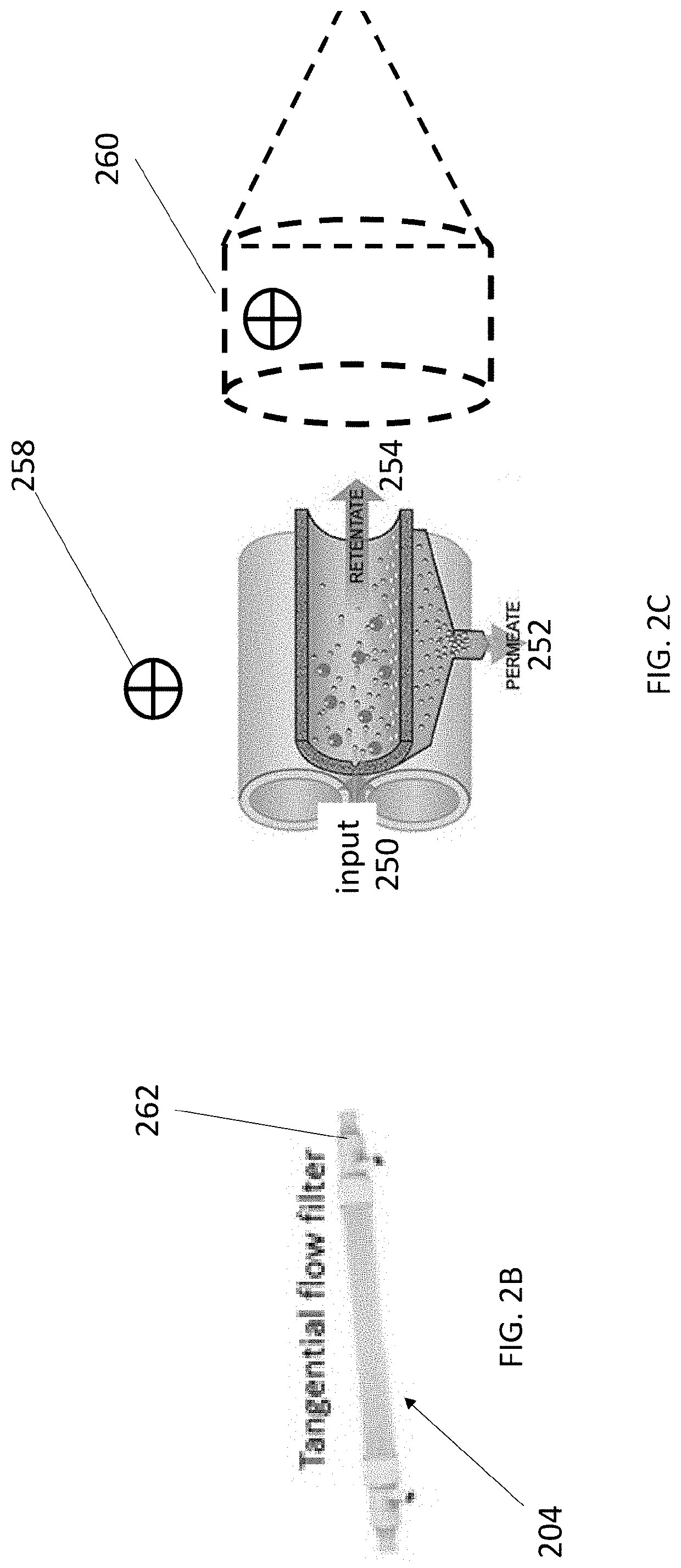
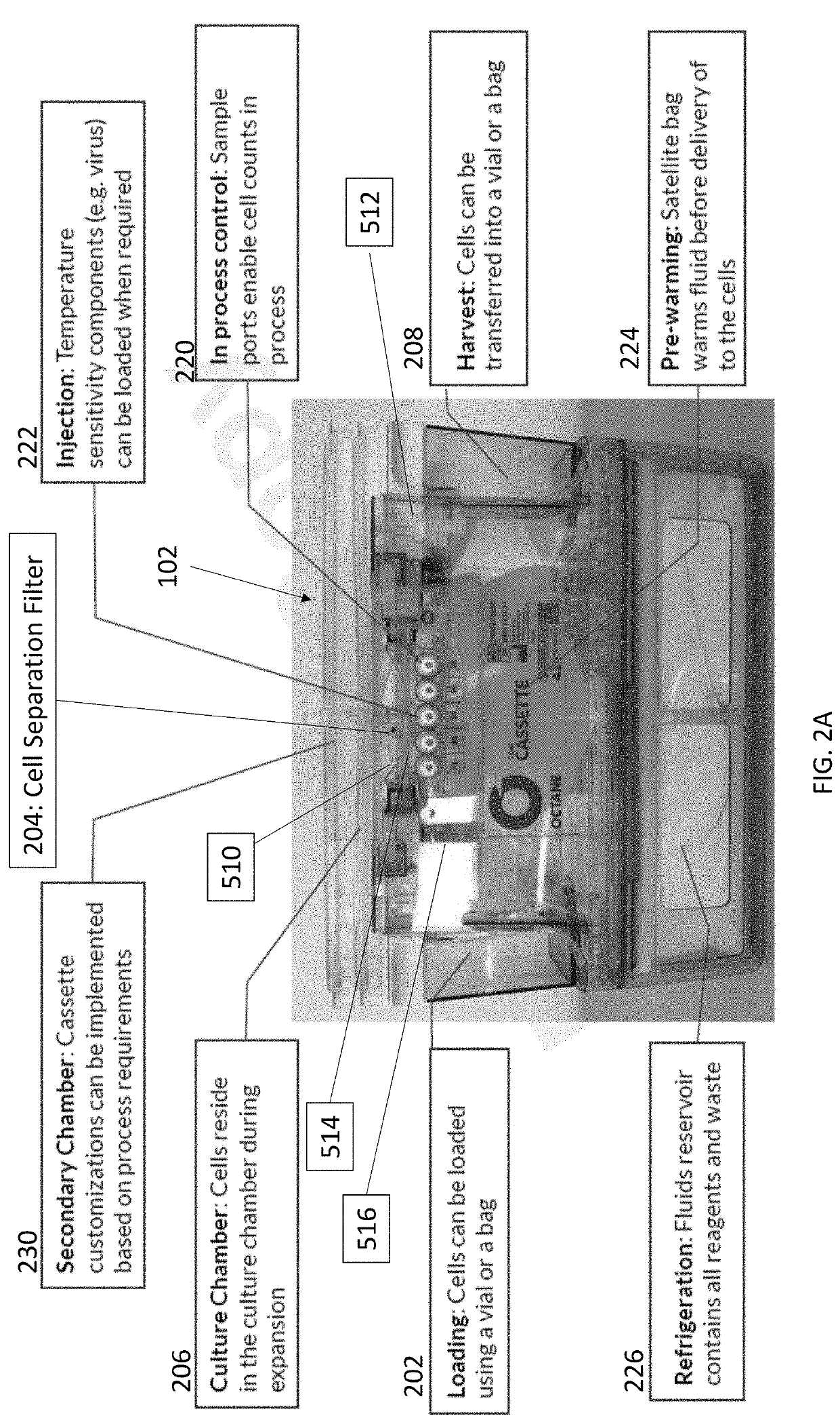
Cell concentration methods and devices for use in automated bioreactors
PendingUS20200255793A1Reduced cell volumeLower the volumeBioreactor/fermenter combinationsMembranesBioreactorCell biology
The present disclosure provides cassettes for use in automated cell engineering systems that include cell concentration filters for reducing fluid volume of a cell sample during or following automated processing. The disclosure also provides methods of concentrating a cell population, as well as automated cell engineering systems that can utilize the cassettes and carry out the methods.
Owner:LONZA WALKERSVILLE INC
Method for effectively inducing cotton somatic embryo and regenerating plants and culture medium thereof
InactiveCN102405835AIncrease incidenceAbility to efficiently regenerateHorticulture methodsPlant tissue cultureMicrobiologyCell engineering
The invention discloses a method for effectively inducing cotton somatic embryo and regenerating plants and culture medium thereof. The culture medium of the invention is applied to the sets of culture medium of the regeneration plants of the cotton somatic cells, and the culture medium comprises culture medium A, culture medium B and culture medium C. The culture medium A comprises basic culture medium of improved MS, hormone, glucose and coagulating agent. The culture medium B comprises the basic culture medium of the improved MS, the hormone, the glucose and amino acid additive. The culture medium C comprises the basic culture medium of the improved MS, the hormone, the glucose, the amino acid additive and the coagulating agent. The method of the invention is a method for cultivating the cotton callus tissues as the regeneration plants by using the sets of the culture medium. The invention cannot be limited by the types of the cotton genes and has short cultivation period and high repeatability, so the cotton effectively obtains a large number of cell embryos and has the ability of regenerating the plants so as to provide an important platform of the researches relative to the cell engineering and the genetic engineering of the cotton and the genetic improvement of the cotton.
Owner:CHINA AGRI UNIV
Agrobacterium-mediated peach genetic transformation method
InactiveCN112695055AIncrease success rateHigh induction ratePlant peptidesGenetic engineeringBiotechnologyGenetic engineering
The invention relates to the field of gardening crop genetic engineering, and provides an agrobacterium-mediated peach genetic transformation method. The method comprises the following steps: establishing a genetic transformation receptor, culturing agrobacterium, preparing an infection solution, conducting infection and co-culturing, conducting degerming, screening and culturing, and carrying out GUS staining identification on resistant calluses. According to the method, the calluses are used as receptors, GUS genes are used as reporter genes, genetic transformation of peaches is carried out with an agrobacterium GV3101 mediation method, the resistant calluses are obtained, and the resistant calluses are determined to be positive calluses through multiple times of subculture and GUS staining. According to the method, the success rate of agrobacterium-mediated transformation can be remarkably increased, and meanwhile, the method can be widely applied to peach genetic engineering, cell engineering, metabolic engineering and establishment of a peach genetic transformation system.
Owner:ANHUI AGRICULTURAL UNIVERSITY
Multistable shape-shifting surfaces
InactiveUS8402711B2Retaining its effectivenessEffective functionProgramme-controlled manipulatorDoor/window protective devicesShape changeEngineering
Multistable shape-shifting surfaces that retain their effectiveness as physical barriers while undergoing changes in shape and that can remain stable in the various shapes. The shape changes include any motion that makes the surface more effective at performing its function, such as expansion, shrinkage, twisting, encircling, wiggling, swallowing or constricting. The shape-shifting surfaces include tiled arrays of polygonal cells, each cell including specifically-designed compliant flexures attached to specifically-shaped overlapping thin plates or shells. The surfaces remain stable by leveraging them during deformation to an extent that they cannot spontaneously return to the unstressed shape. Applications for such surfaces include micro-scale cellular engineering and macro-scale biomedical applications, recreational uses, national security, and environmental protection.
Owner:UNIV OF SOUTH FLORIDA
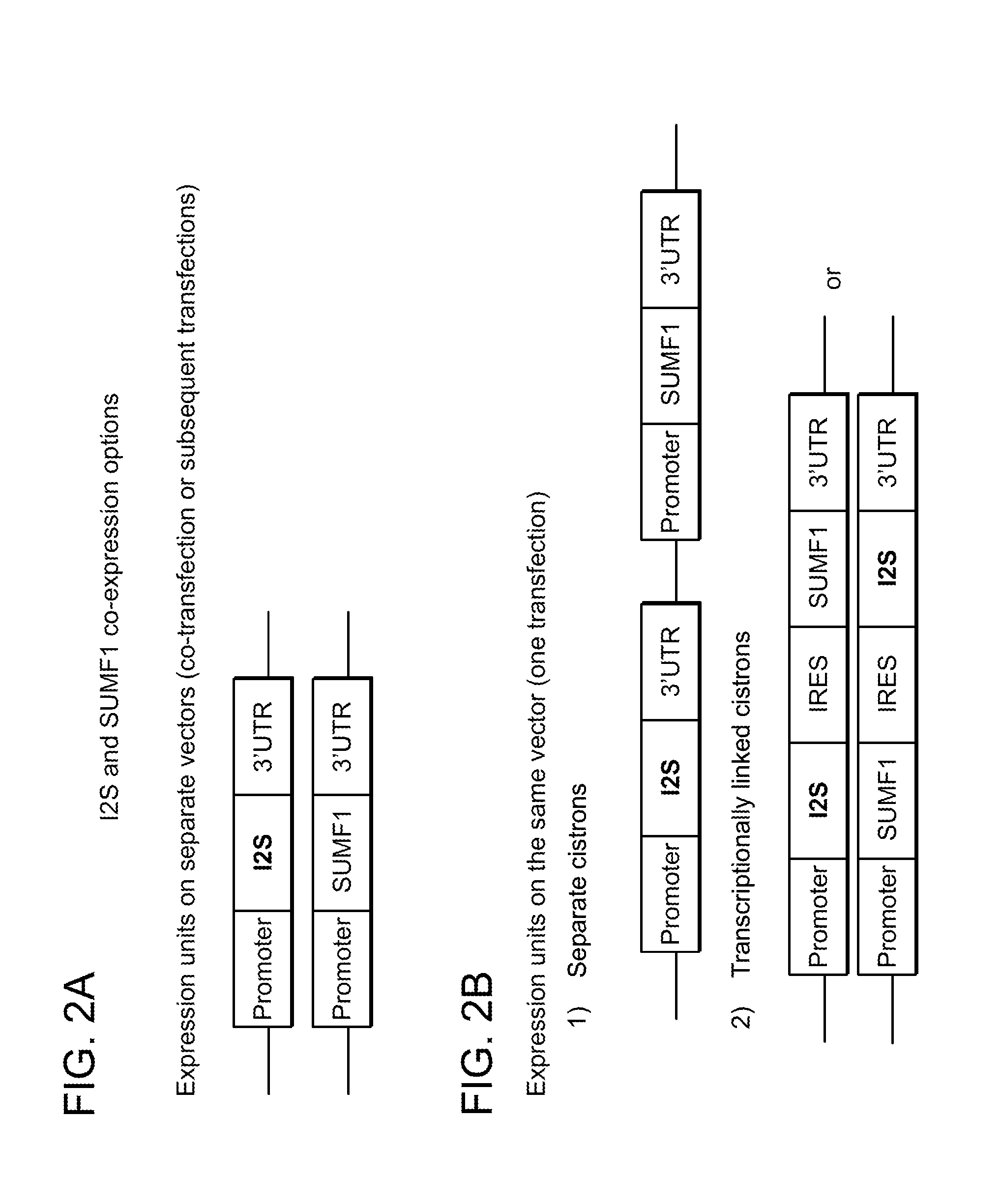
Cells for producing recombinant iduronate-2-sulfatase
ActiveUS9150841B2Effective enzyme replacement therapyImprove the level ofPeptide/protein ingredientsHydrolasesIduronate-2-sulfataseSerum free
The present invention provides, among other things, methods and compositions for production of recombinant I2S protein with improved potency and activity using cells co-express I2S and FGE protein. In some embodiments, cells according to the present invention are engineered to simultaneously over-express recombinant I2S and FGE proteins. Cells according to the invention are adaptable to various cell culture conditions. In some embodiments, cells of the present invention adaptable to a large-scale suspension serum-free culture.
Owner:TAKEDA PHARMA CO LTD
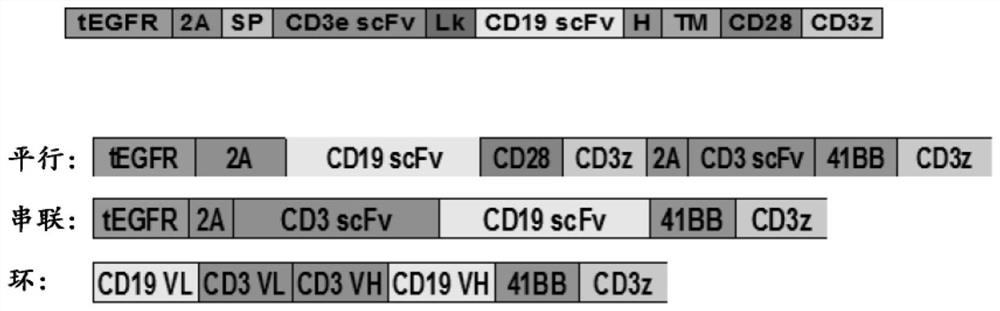
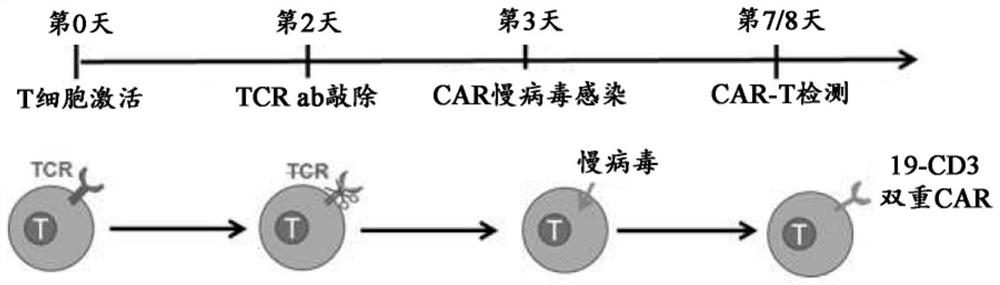
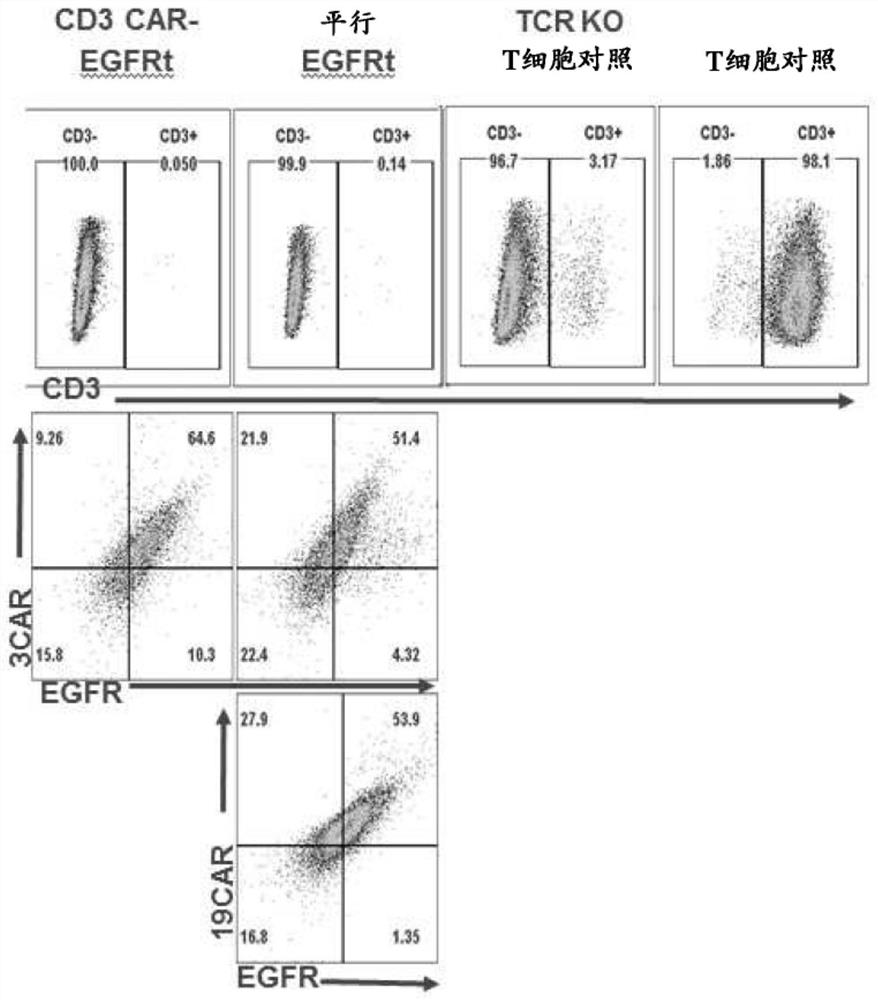
Compositions and methods for t cell engineering
PendingCN113383071AVirusesPeptide/protein ingredientsNatural Killer Cell Inhibitory ReceptorsAntigen binding
The disclosure relates to an engineered immune cell and use thereof. The engineered immune cell provided in the disclosure comprises a CAR or engineered TCR, which CAR or engineered TCR can comprise a first antigen binding domain and a second antigen binding domain. The engineered immune cells, when administered into a subject, can inhibit the host immune cells such as T cells and / or NK cells and enhance the survival and persistence of the engineered immune cells in vivo, thereby exhibiting more effective tumor killing activity.
Owner:GRACELL BIOTECH SHANGHAI CO LTD +1
Immune effector cell engineering and use thereof
PendingUS20210163622A1Cut surfaceIncreasing tumor cell surface MICA/B densityHydrolasesAntibody mimetics/scaffoldsDirected differentiationImmune effector cell
Provided are methods and compositions for obtaining functionally enhanced derivative effector cells obtained from directed differentiation of genomically engineered iPSCs. The derivative cells provided herein have stable and functional genome editing that delivers improved or enhanced therapeutic effects. Also provided are therapeutic compositions and the used thereof comprising the functionally enhanced derivative effector cells alone, or with antibodies or checkpoint inhibitors in combination therapies.
Owner:FATE THERAPEUTICS
Cell isolation for use in automated bioreactors
InactiveUS20200181562A1Bioreactor/fermenter combinationsBiological substance pretreatmentsEngineeringCell isolation
The present disclosure provides cassettes for use in automated cell engineering systems that include cell separation filters for capturing a target cell population for automated processing. The disclosure also provides methods of separating a target cell population, as well as automated cell engineering systems using the cassettes and for carrying out the methods.
Owner:LONZA WALKERSVILLE INC
Poplar somatic embryo generation and plant construction method
ActiveCN108575740AIncrease productionShort training periodPlant tissue cultureHorticulture methodsGeneration ratePlant cell
The invention discloses a poplar high efficiency somatic embryo generation and plant construction method, and more specifically provides a polar tissue culture method. The poplar somatic embryo generation and plant construction method comprises following steps: 1, an induction medium (containing 1.0 to 2.0mg / L 2,4-D and 0.1mg / L KT) is inoculated with an explants of polar for culturing so as to obtain callus; a differentiation medium is inoculated with the callus for culturing so as to obtain adventitious buds; 3, a rooting medium is inoculated with the adventitious buds for culturing so as toobtain seedlings with roots. The polar tissue culture method possesses following advantages: the culture period is short; the repeatability is high; different hormone combinations are adopted, so thatpoplar embryo generation rate is increased; and a large amount of regenerated plants can be obtained. The polar tissue culture method is capable of providing an ideal experiment system for deep research of plant cell differentiation, and an important platform for polar cell engineering, genetic engineering, and polar genetic improvement.
Owner:BEIJING FORESTRY UNIVERSITY
Rapid and efficient cymbopogon citratus protoplast preparation method
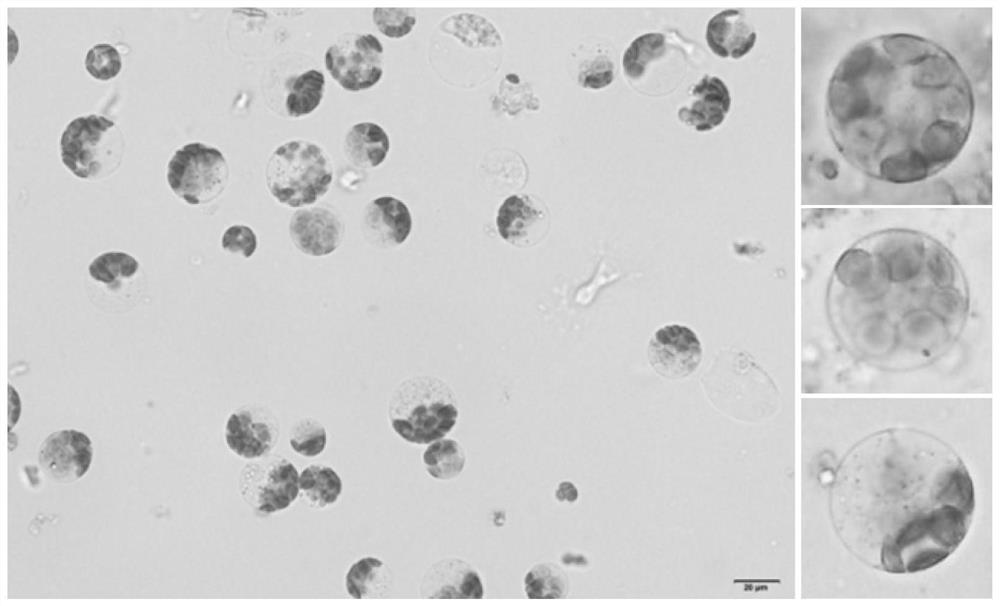
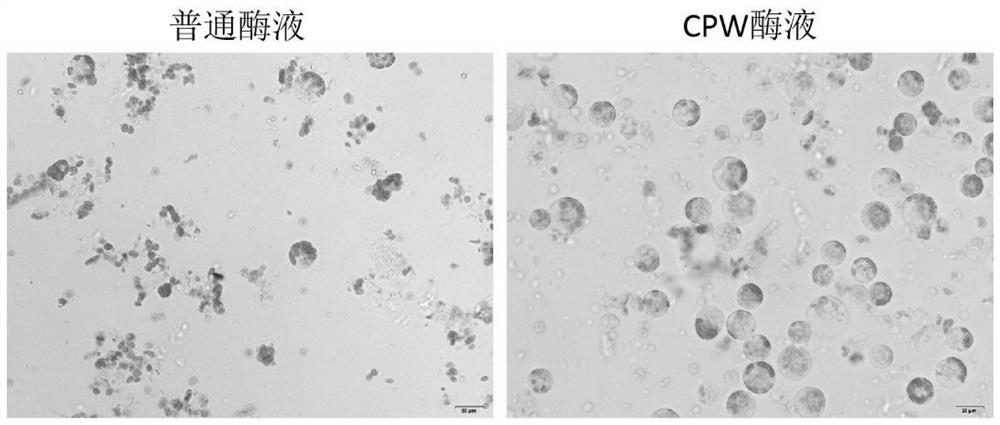
The invention discloses a rapid and efficient cymbopogon citratus protoplast preparation method, and belongs to the field of plant cell engineering. Healthy-growing cymbopogon citratus tissue culture seedlings are adopted as experimental materials, a CPW solution is used for preparing enzymatic hydrolysate, a low-speed oscillation enzymolysis method is adopted for carrying out enzymolysis digestion on cymbopogon citratus leaves, and the protoplast is efficiently prepared. And finally, purifying and collecting of the high-quality cymbopogon citratus protoplast is conducted by using a sucrose density gradient centrifugation method. The method is simple, convenient and easy to operate, and the separated protoplast is high in yield, complete in cell, spherical, rich in chloroplast and relatively high in activity. The invention provides an important basis for research and application in the aspects of gene function identification, subcellular localization, protein interaction, citronella cell fusion, single cell sequencing and the like by utilizing a cymbopogon citratus leaf instantaneous transformation system.
Owner:SHANGHAI CHENSHAN BOTANICAL GARDEN
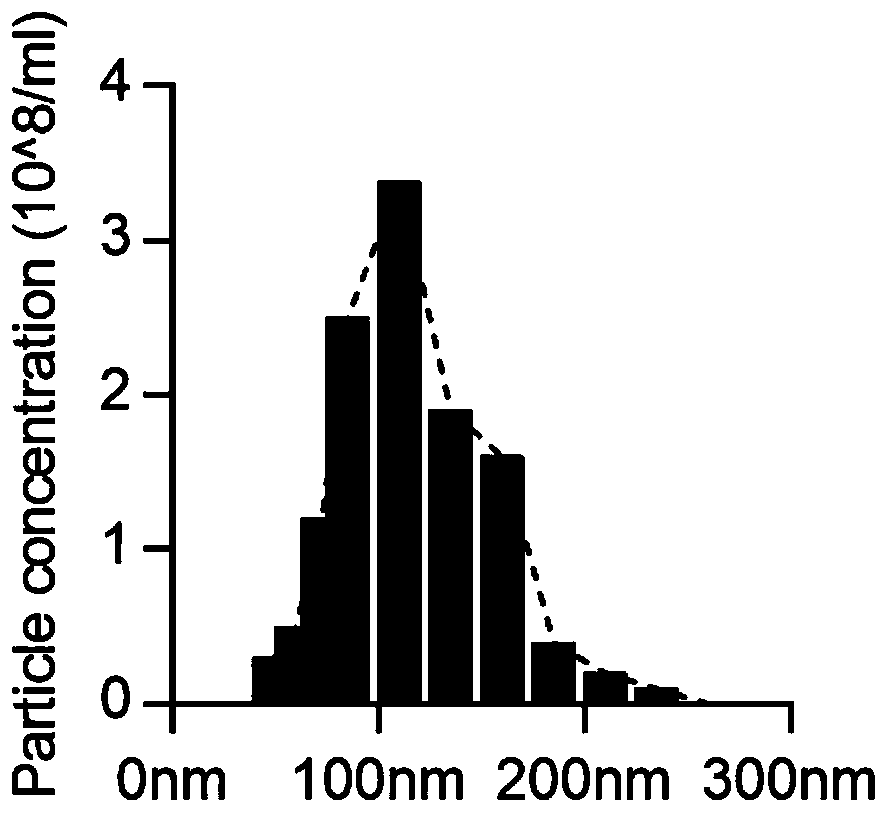
Brown-like fat exosome and engineering production method and application thereof
The invention provides a brown-like fat exosome and an engineering production method and application thereof, and belongs to the technical field of cell engineering and gene recombination. The engineering production method of the brown-like adipose exosome comprises the following steps: separating adipose-derived stem cells from adipose tissues; constructing a virus expression vector for simultaneously expressing PGC1a and the PRDM16; packaging the virus expression vector into a virus; infecting the adipose-derived stem cells with the virus; culturing the adipose-derived stem cells infected with the virus, and collecting the brown-like fat exosome. Through the genetic modification method, pGC1a and PRDM16 genes are introduced into adipose-derived stem cells to activate the expression of aplurality of brown fat-related genes in the adipose-derived stem cells, the adipose-derived stem cells are modified into brown adipose-derived stem cells, and then the brown-like fat exosome is generated. The brown-like fat exosome has characteristics of brown fat exosomes, and a potential treatment means is provided for metabolic diseases such as obesity.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Method for building Chinese cabbage genetic transformation system by cell-penetrating peptide and microspore culture
InactiveCN104818296AQuickly obtain target traitsShorten the breeding periodOther foreign material introduction processesPlant tissue cultureBiotechnologyGenetics genomics
The invention belongs to the field of a plant cell engineering technology and molecular biology and relates to a method for building a Chinese cabbage genetic transformation system by cell-penetrating peptide and microspore culture. The method comprises carrying out in-vitro synthesis of a transfection complex by film-penetrating functions of cell-penetrating peptide, adding the transfection complex into a separated microspore suspension liquid, preparing a regeneration plant by a free microspore culture technology and carrying out identification on the regeneration plant. The method builds the Chinese cabbage genetic transformation system based on free microspore culture, provides a study platform for Chinese cabbage genetic breeding and lays the method foundation of Chinese cabbage functional genomics.
Owner:SHENYANG AGRI UNIV
Cherry valley duck embryo epithelial cell line and establishment method thereof
ActiveCN103725644ALow nutritional requirementsReduce the cost of trainingEmbryonic cellsSurvivabilityCulture fluid
The invention discloses a cherry valley duck embryo epithelial cell line and an establishment method thereof, and belongs to the field of cytobiology. The method comprises the steps of taking duck embryo tissue with 9 to 12 age in days in a sterile way, shearing the duck embryo tissue into tissue blocks, adhering the tissue blocks downwards to the bottom of a culture hole, adding nutrient solution into a carbon dioxide incubator, and performing plate adhering culture at 37 DEG C. The high-survivability and high-purity cherry valley duck embryo epithelial cell line is obtained according to the method and conducted to biological preservation, compared with primary cell, the cell line has the advantages that the cellular morphology and the physiological property of the cell line are obvious different from that of the primary cell, continuous and stable passage can be realized, the nutritional requirement is low, the growth cycle is short, and the cell line is suitable for large-scale culture, provides a large amount of high-quality cell material for life sciences, such as gene engineering, cell engineering, immunology, molecular biology and the like, can serve as donor cell for poultry somatic cell clone breeding, and can also serve as a main material for duck virus isolation and vaccine production.
Owner:SHANDONG LVDU BIO SICIENCE & TECH
Automated production of viral vectors
The present disclosure provides an automated method of producing viral vectors, utilizing engineered viral vector-producing cell lines within a fully-enclosed cell engineering system. Exemplary viral vectors that can be produced include lentivirus vectors, adeno-associated virus vectors, baculovirus vectors and retrovirus vectors.
Owner:LONZA WALKERSVILLE INC
Reagents and methods for replication, transcription, and translation in semi-synthetic organisms
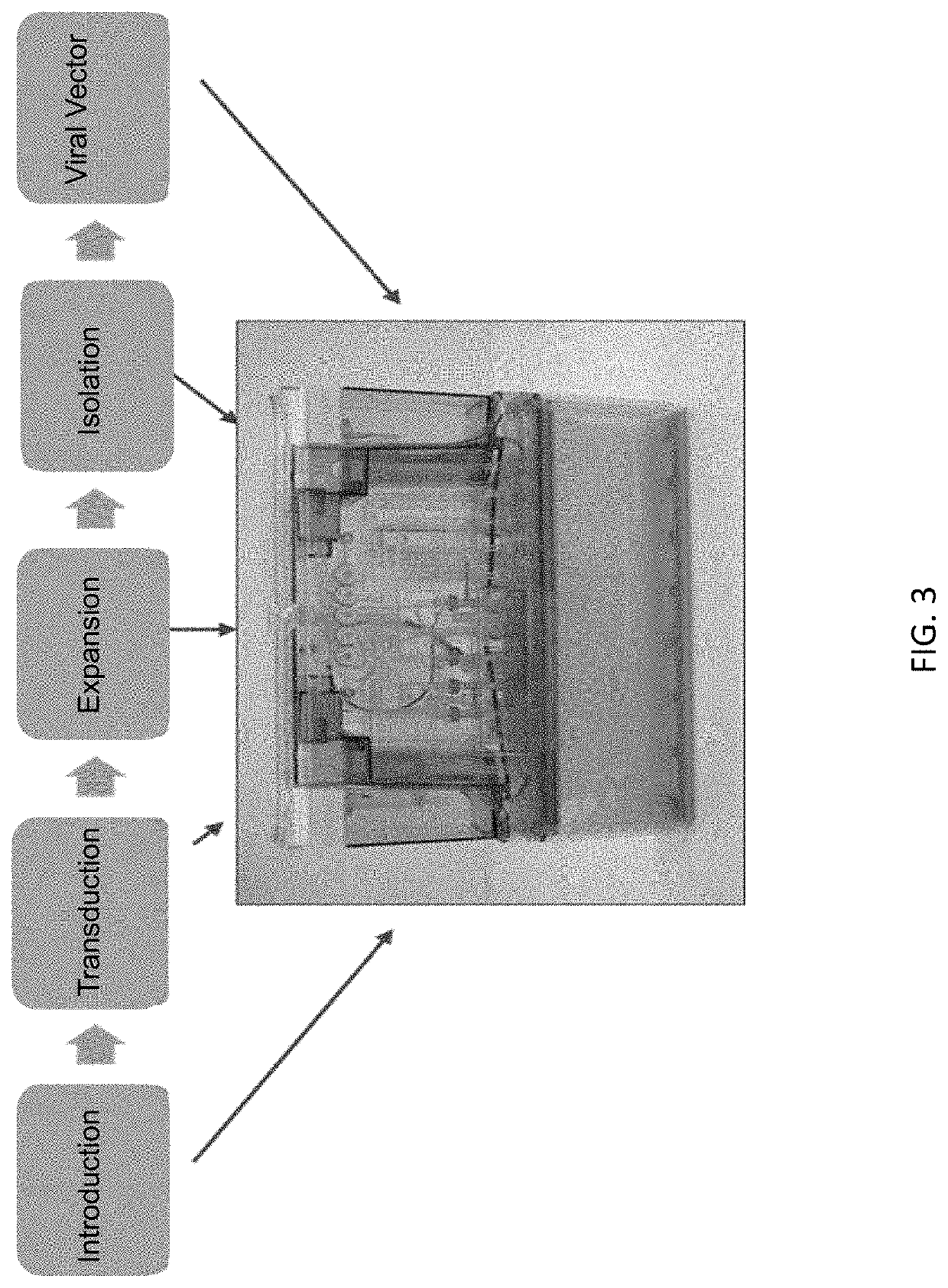
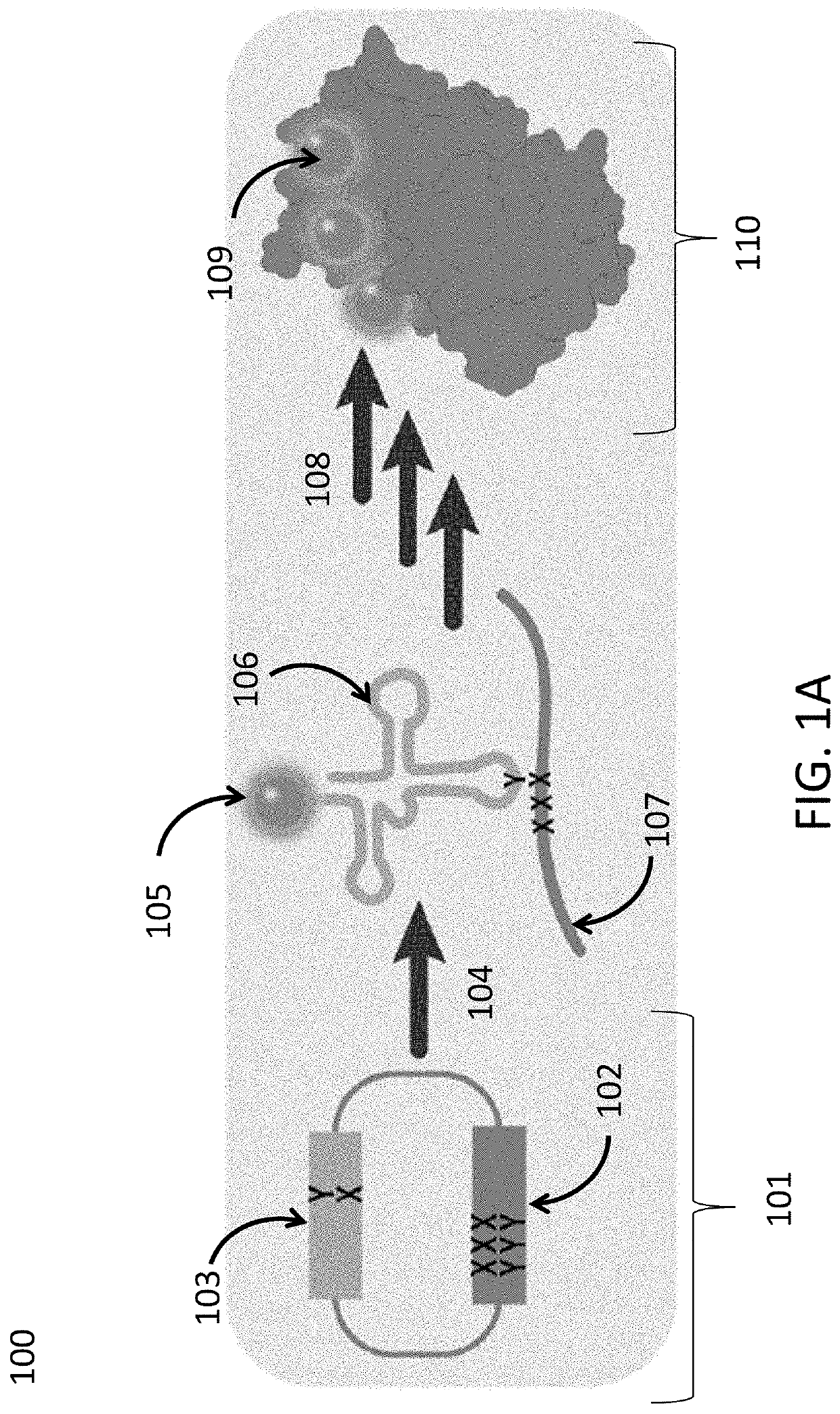
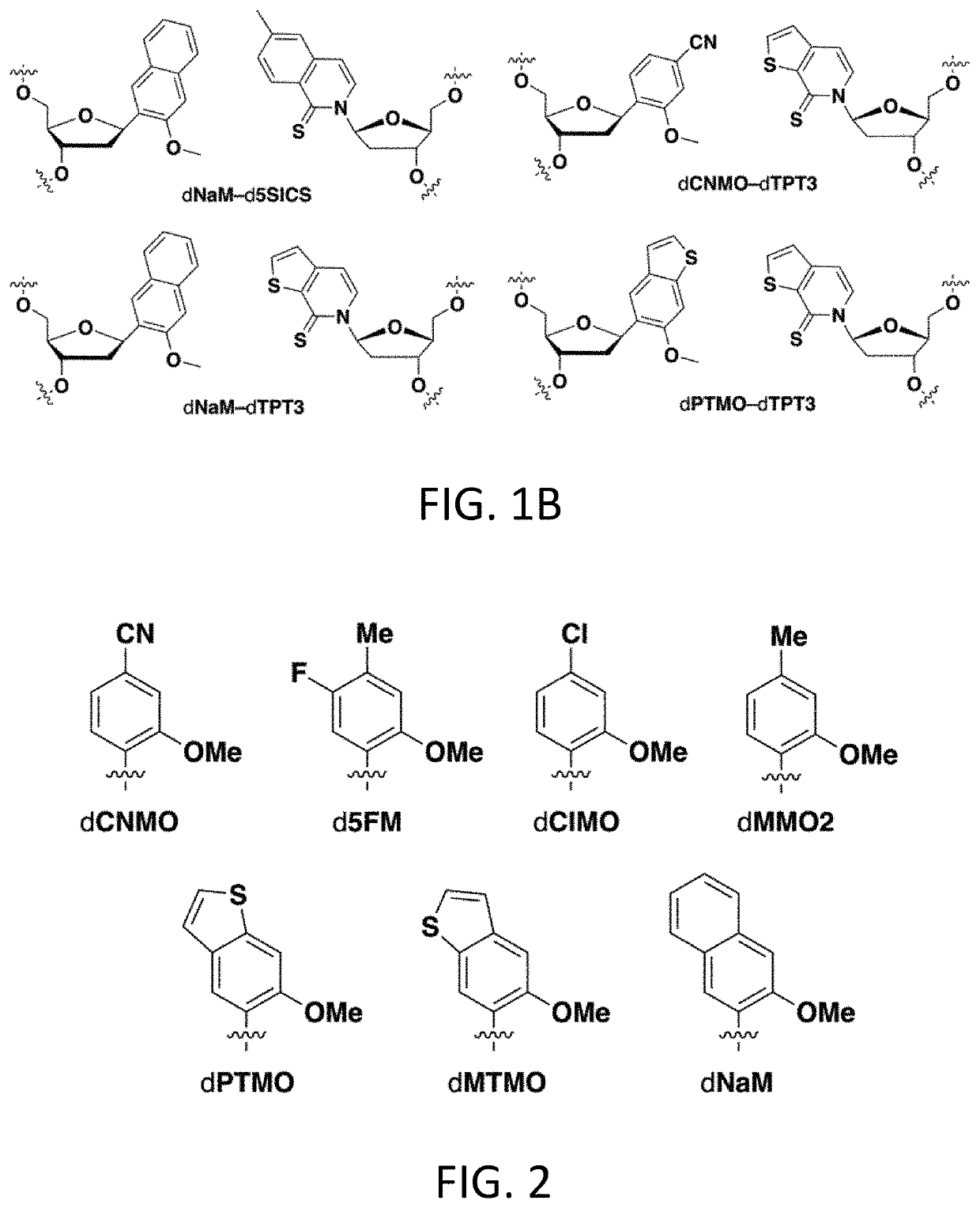
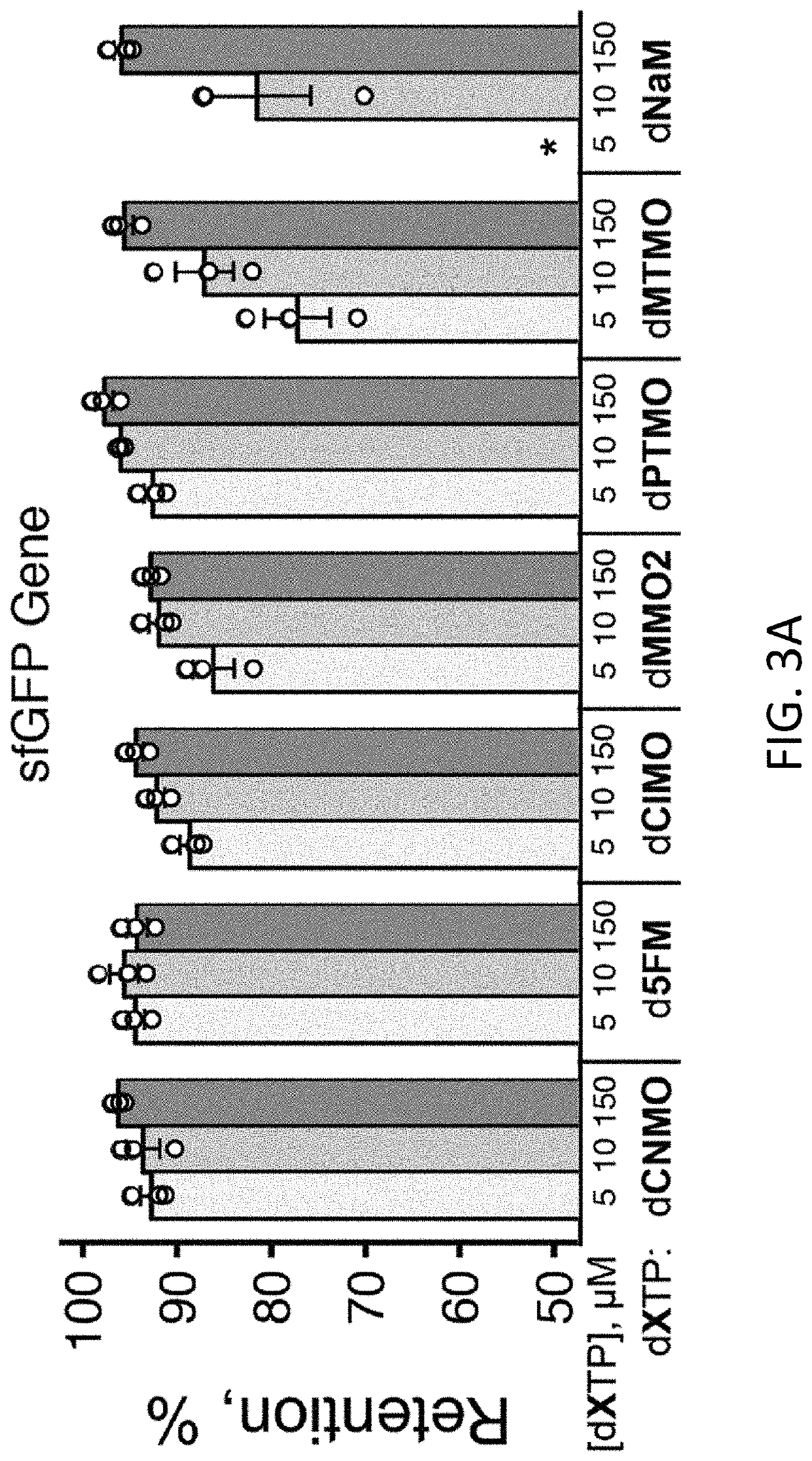
Disclosed herein are compositions, methods, cells, engineered microorganisms, and kits for increasing the production of proteins or polypeptides comprising one or more unnatural amino acids. Further provided are compositions, cells, engineered microorganisms, and kits for increasing the retention of unnatural nucleic acids encoding the unnatural amino acids in an engineered cell, or semi-synthetic organism.
Owner:THE SCRIPPS RES INST
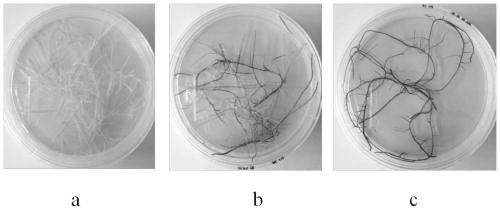
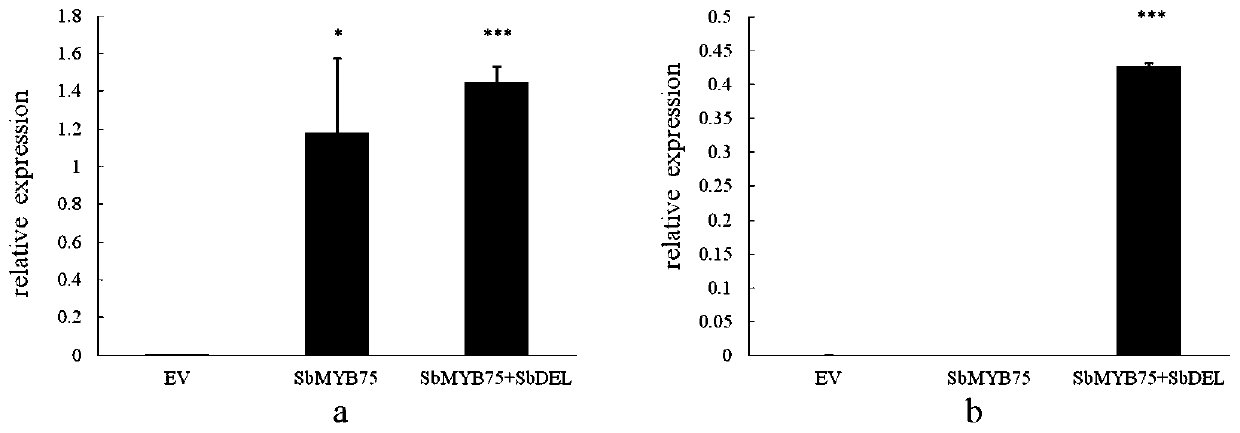
Gene cloning, vector construction and application of baical skullcap root anthocyanin transcriptional regulation factors SbMYB75 and SbDEL
The invention discloses a baical skullcap root anthocyanin transcriptional regulation factor, which comprises at least one of a SbMYB75 gene and a SbDEL gene, wherein a SbMYB75 gene sequence is shownas SEQ ID No.1, and a SbDEL gene sequence is shown as SEQ ID No.3. The invention also provides a primer composition for amplifying the above genes, a protein encoded by the genes, a recombinant vector, a recombinant microorganism, a host cell, a transgenic cell line or a transgenic plant tissue constructed by the above genes and an application thereof. Baical skullcap root anthocyanin transcription factors SbMYB75 and SbDEL and the recombinant vector constructed by the baical skullcap root anthocyanin transcription factors SbMYB75 and SbDEL are utilized to improve anthocyanin yield of the transgenic cell line. According to the gene cloning, vector construction and the application of the baical skullcap root anthocyanin transcriptional regulation factors SbMYB75 and SbDEL, the anthocyanin transcription factors SbMYB75 and SbDEL and encoded proteins thereof are cloned from baical skullcap root for the first time, the transgenic cell lines with high anthocyanin content are obtained through genetic engineering and cell engineering, and a solid foundation is laid for industrial improvement of the anthocyanin yield.
Owner:SHANGHAI CHENSHAN BOTANICAL GARDEN
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com