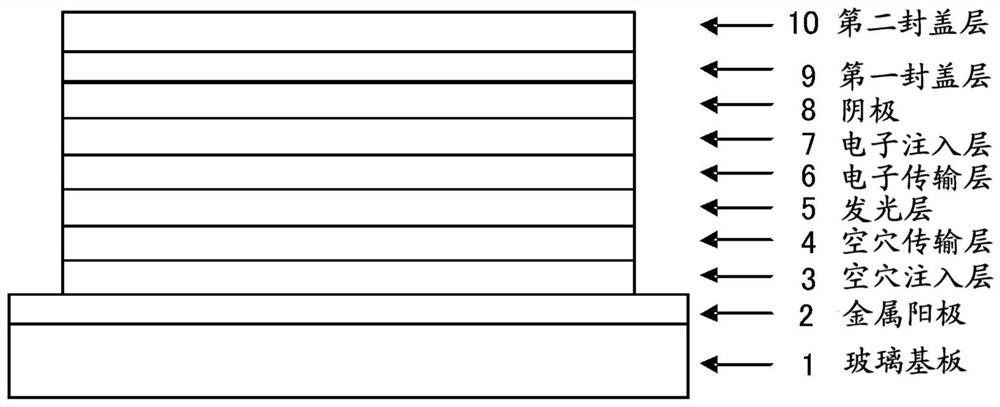
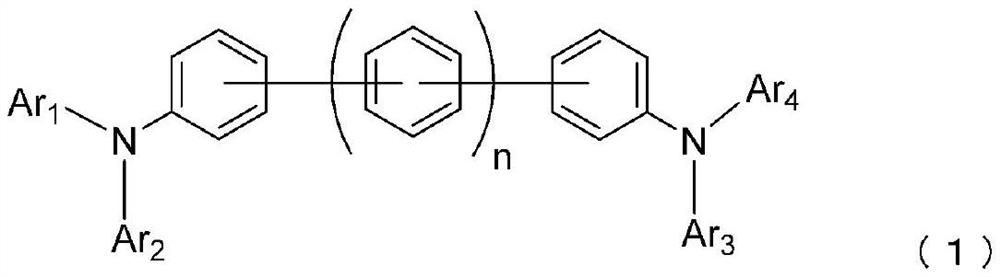
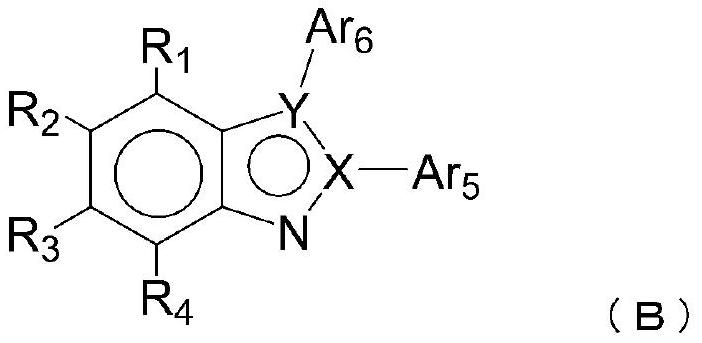
Organic electroluminescence element
An electroluminescent element and organic technology, applied in the direction of organic light emitting devices, organic light emitting device parameters, electroluminescent light sources, etc., can solve problems such as poor alignment accuracy and reduced color purity extraction efficiency, and achieve improved extraction efficiency, The effect of optimizing the extraction efficiency and high refractive index
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0167]
[0168] 4.2 g of 2-(4-bromophenyl)-2H-benzo[1,2,3]triazole, 2.3 g of N,N'-diphenylbenzidine, tert-butyl Sodium oxide 2.0g, toluene 50ml, while irradiating ultrasonic waves for 30 minutes, nitrogen gas was passed through. 62.0 mg of palladium acetate and 0.2 ml of tri-tert-butylphosphine were added, heated, and stirred at 91° C. for 5 hours. After cooling to room temperature, 50 ml of toluene was added and an extraction operation was performed to obtain an organic layer. After concentrating the organic layer, it was purified by column chromatography (carrier: NH silica gel, eluent: toluene / n-hexane), and further, by performing dispersion washing using 100 ml of n-hexane, N,N'-bis{4 -(2H-benzo[1,2,3]triazol-2-yl)phenyl}-N,N'-diphenyl-4,4'-diamino-1,1'-biphenyl (compound (1-1)) yellow powder 3.3g (yield 66%).
[0169] The structure of the obtained yellow powder was identified using NMR.
[0170] use 1 H-NMR (THF-d 8 ) detected the following 34 hydrogen signals.
...
Embodiment 2
[0175]
[0176] Add 14.0 g of 4,4"-diiodo-1,1':4',1"-terphenyl and {4-(2H-benzo[1,2,3]triazole to the nitrogen-substituted reaction vessel -2-yl)phenyl}phenylamine 18.3g, potassium carbonate 13.2g, copper powder 0.3g, sodium bisulfite 0.9g, 3,5-di-tert-butylsalicylic acid 0.7g, dodecane 30 ml of phenylbenzene was heated and stirred at 210° C. for 44 hours. After naturally cooling to room temperature, 50 ml of toluene was added, and the precipitate was obtained by filtration. 230 ml of 1,2-dichlorobenzene was added to the precipitate and heated to dissolve it, and the insoluble matter was removed by hot filtration. The filtrate was concentrated, purified by crystallization using 1,2-dichlorobenzene, and then dispersed and washed with methanol to obtain N,N'-bis{4-(2H-benzo[1,2 , 3] triazol-2-yl) phenyl}-N, N'-diphenyl-4,4"-diamino-1,1': 4', 1"-terphenyl (compound (1-2 )) yellow powder 22.2g (96% yield).
[0177] The structure of the obtained yellow powder was identified u...
Embodiment 3
[0183]
[0184] In Example 3, instead of {4-(2H-benzo[1,2,3]triazol-2-yl)phenyl}phenylamine, {4-(benzoxazol-2-yl) Phenyl}phenylamine, by reacting under the same conditions, N,N'-bis{4-(benzoxazol-2-yl)phenyl}-N,N'-diphenyl - 12.4 g of yellow powder of 4,4"-diamino-1,1': 4',1"-terphenyl (compound (1-22)) (yield 47%).
[0185] The structure of the obtained yellow powder was identified using NMR.
[0186] use 1 H-NMR (CDCl 3 ) detected the following 38 hydrogen signals.
[0187] δ(ppm)=8.13(4H), 7.80-7.55(11H), 7.50-7.16(23H)
[0188] [chemical 15]
[0189]
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap