Bi-specific extracellular matrix binding peptides and methods of use thereof
An amino acid and sequence technology, applied in the field of cancer treatment and molecular medicine, can solve difficult problems and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0558] Example 1: Selection and Characterization of PL1 and Related Peptides
[0559] A. Materials and methods
[0560] 1. Materials
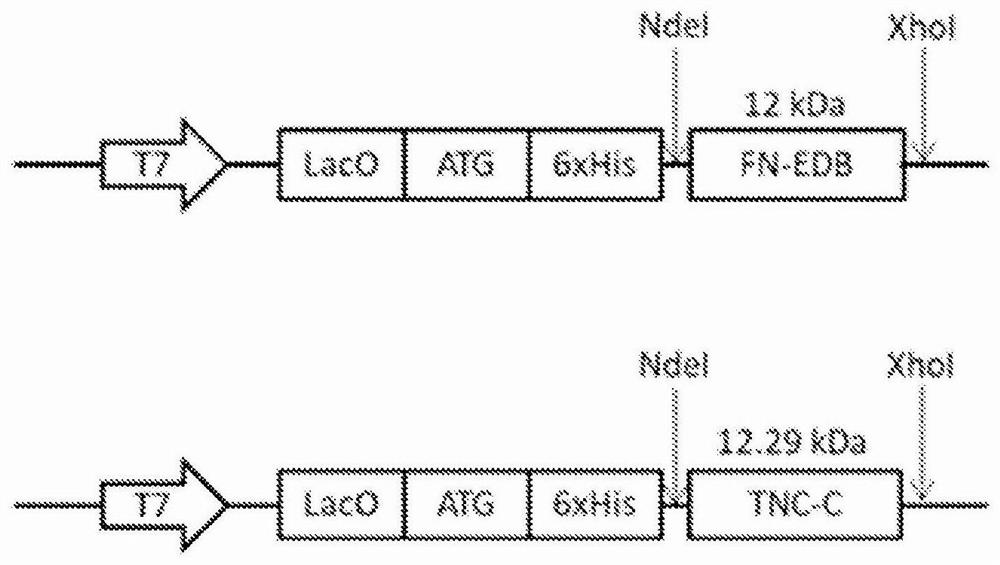
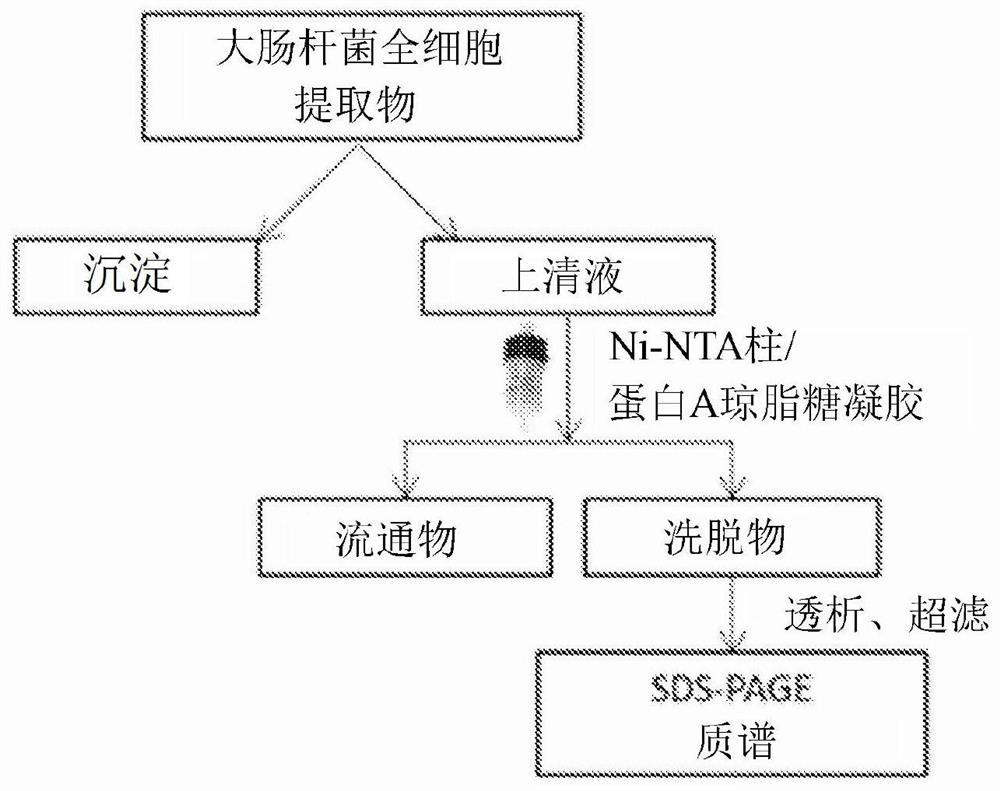
[0561] Phosphate-buffered saline (PBS) was purchased from Lonza (Verviers, Belgium), K 3 [Fe(CN) 6 ], HCl, Nuclear Fast Red, Xylene Substitute, Isopropanol, Triton-X, Tween 20, CHCl 3 , MeOH and dimethylformamide (DMF) were purchased from Sigma-Aldrich (Munich, Germany). Cloning, expression, purification of proteins (FN-EDB, TNC-C, NRP1, NCL, and single-chain antibodies FN-EDB-L19 and TNC-C-G11), and generation of polyclonal rabbit antibodies are described below and in Table 1 .
[0562] Table 1: List of antibodies used
[0563]
[0564]
[0565] 2. Cell lines
[0566] U87MG GBM and PC3 prostate cancer cells were obtained from ATCC, and NCH421K cells were obtained from CLS Cell Lines Service GmbH (Eppelheim, Germany). WT-GBM and VEGF-KO GBM cells were a gift from Gabriele Bergers (Leuven, Belgium).
[0567] 3. Clinical samples
...
Embodiment 2
[0659] Example 2: Selection and Characterization of PL2 Peptides and Related Peptides
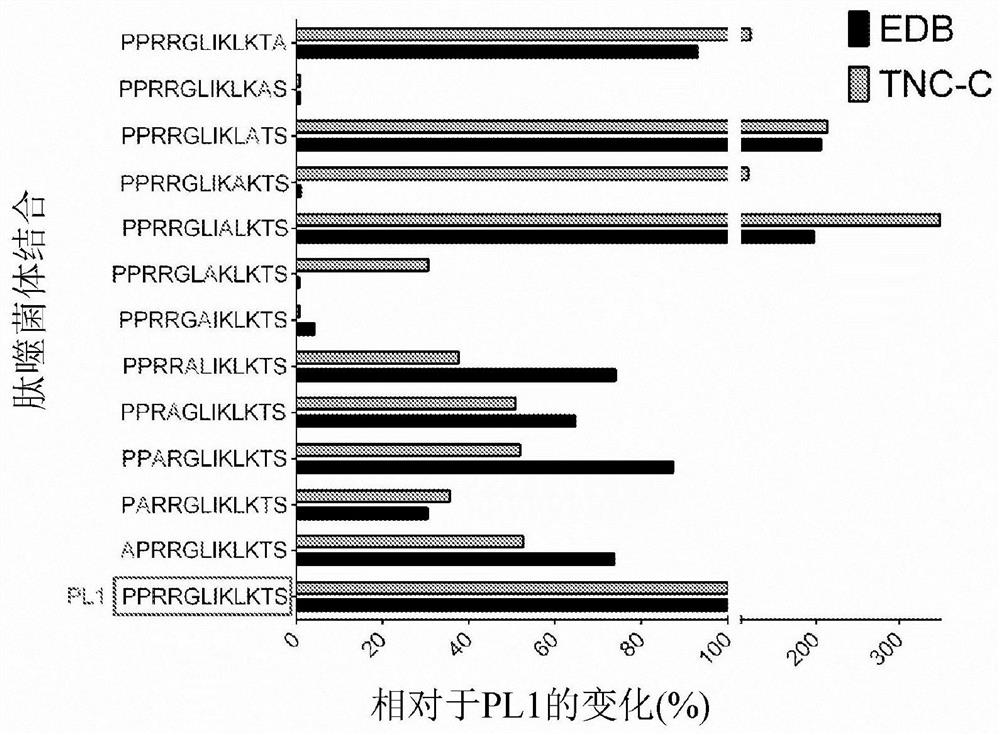
[0660] Peptides were generally selected and tested for binding to FN-EDB using techniques and protocols as described in Example 1. The data and results are shown in Figures 3 to 5 and 22 in.
[0661] A. Materials and methods
[0662] 1. Materials
[0663] Phosphate buffered saline (PBS) was purchased from Lonza (Verviers, Belgium). K 3 [Fe(CN) 6 ], HCl, Isopropanol, Triton-X, Tween 20, CHCl 3 , MeOH, isopropyl β-D-1-thiogalactoside (IPTG) and dimethylformamide (DMF) were purchased from Sigma-Aldrich (Munich, Germany).
[0664] 2. Peptides and proteins
[0665] Cys-5(6)-carboxyfluorescein (FAM)-PL2 with a 6-aminocaproic acid spacer and Cys-FAM peptide were purchased from TAGCopenhagen (Denmark). Plasmids pASK75-Fn7B8 and pASK75-Fn789 were kindly provided by Dr. Ame Skerra (Schiefner et al., 2012). Amplify the gene fragment of the Fn-EDB domain from a plasmid and clone it into the N-...
Embodiment 3
[0703] Example 3: Selection and Characterization of PL3 Peptides and Related Peptides
[0704] Peptides were generally selected and tested for binding to TNC-C using techniques and protocols as described in Example 1. Some of the data and results are shown in Figures 6 to 8 middle.
[0705] A. Materials and methods
[0706] 1. Materials
[0707] Phosphate buffered saline (PBS) was purchased from Lonza (Verviers, Belgium). K 3 [Fe(CN) 6 ], HCI, Isopropanol, Triton-X, Tween 20, CHCl 3 , MeOH, isopropyl β-D-1-thiogalactoside (IPTG) and dimethylformamide (DMF) were purchased from Sigma-Aldrich (Munich, Germany). Cys-fluorescein (FAM)-PL3 and Cys-FAM peptides with a 6-aminocaproic acid spacer were purchased from TAG Copenhagen (Denmark). Synthesized on a microwave-assisted automated peptide synthesizer (Liberty; CEM Corporation, Matthews, NC, USA) using Fmoc / t-Bu chemistry [ D (KLAKLAK) 2 ]with[ D (KLAKLAK) 2 ] - PL3 peptide (SEQ ID NO: 6). Peptides were purified to 9...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com