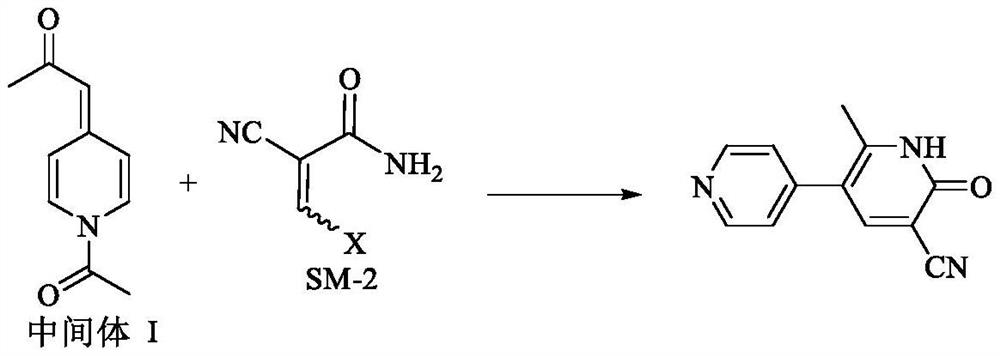
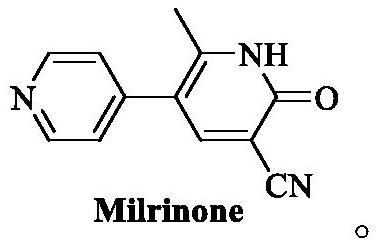
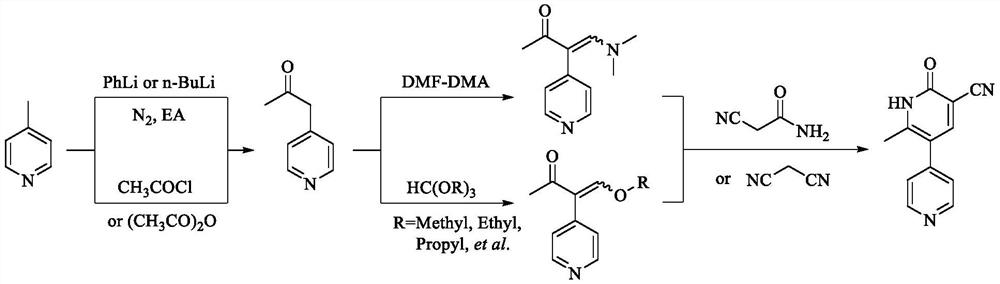
Preparation process of milrinone
A technology of milrinone and intermediates, applied in the field of drug synthesis, can solve the problems of low operation safety, difficult operation, harsh reaction conditions, etc., and achieve the effects of shortening the process route, easy control of the feeding amount, and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] Example 1 Preparation of 1-(1-acetylpyridine-4(1H)-ylidene)-2-propanone
[0081] Add 4-picoline (93.08g, 1.0mol) into dichloromethane (200ml), and add acetyl chloride (172.72g, 2.2mol) at a temperature of 15-20°C. After 1 hour, the reaction is complete; temperature control is 10-15° C., adding purified water (400 ml), adding sodium hydroxide solution (about 7.5 mol / ml) while stirring to adjust the pH value of the reaction solution to 13-14, separating the liquids, and collecting the organic phase. Extract the aqueous phase with dichloromethane (100ml×2), combine the organic phases, dry over anhydrous sodium sulfate, filter, control the temperature of the filtrate at 50-60°C and distill under reduced pressure until no fraction flows out, and continue to heat up the concentrated solution at 70-80°C to concentrate under reduced pressure , to obtain viscous liquid (recovery 4-picoline 55.85g, recovery rate 60%); add ethyl acetate (45ml) to viscous liquid, add acetic anhydri...
Embodiment 2
[0082] Example 2 Preparation of 1-(1-acetylpyridine-4(1H)-ylidene)-2-propanone
[0083] Add 4-methylpyridine (93.12g, 1.0mol) into dichloromethane (150ml), and add acetyl chloride (94.24g, 1.2mol) at a temperature of 20-25°C. After the addition is complete, control the temperature at 20-30°C to react 6 After 1 hour, the reaction is complete; temperature control is 0~10 DEG C, add purified water (400ml), while stirring, add sodium hydroxide solution (about 7.5mol / ml) to adjust the pH value of the reaction solution to 13~14, separate the liquid, collect the organic phase, Extract the aqueous phase with dichloromethane (100ml×2), combine the organic phases, dry over anhydrous sodium sulfate, filter, control the temperature of the filtrate at 50-60°C and distill under reduced pressure until no fraction flows out, and continue to heat up the concentrated solution at 70-80°C to concentrate under reduced pressure , to obtain viscous liquid (recovery 4-picoline 57.73g, recovery rate 6...
Embodiment 3
[0084] Example 3 Preparation of 1-(1-acetylpyridin-4(1H)-ylidene)-2-propanone
[0085] Add 4-methylpyridine (93.07g, 1.0mol) into dichloromethane (300ml), add acetyl chloride (235.54g, 3mol) under temperature control at 20-25°C, and react for 5 hours under temperature control at 20-30°C , the reaction is complete; add purified water (500ml) at 15-20°C with temperature control, add sodium hydroxide solution (about 7.5mol / ml) while stirring, adjust the pH value of the reaction solution to 13-14, separate the liquids, collect the organic phase, water Extract with dichloromethane (150ml×2), combine the organic phases, dry over anhydrous sodium sulfate, filter, control the temperature of the filtrate at 50-60°C and distill under reduced pressure until no fraction flows out, continue to heat up the concentrated solution at 70-80°C and concentrate under reduced pressure. A viscous liquid was obtained (54.91 g of 4-picoline was recovered, the recovery rate was 59%); ethyl acetate (50 ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap