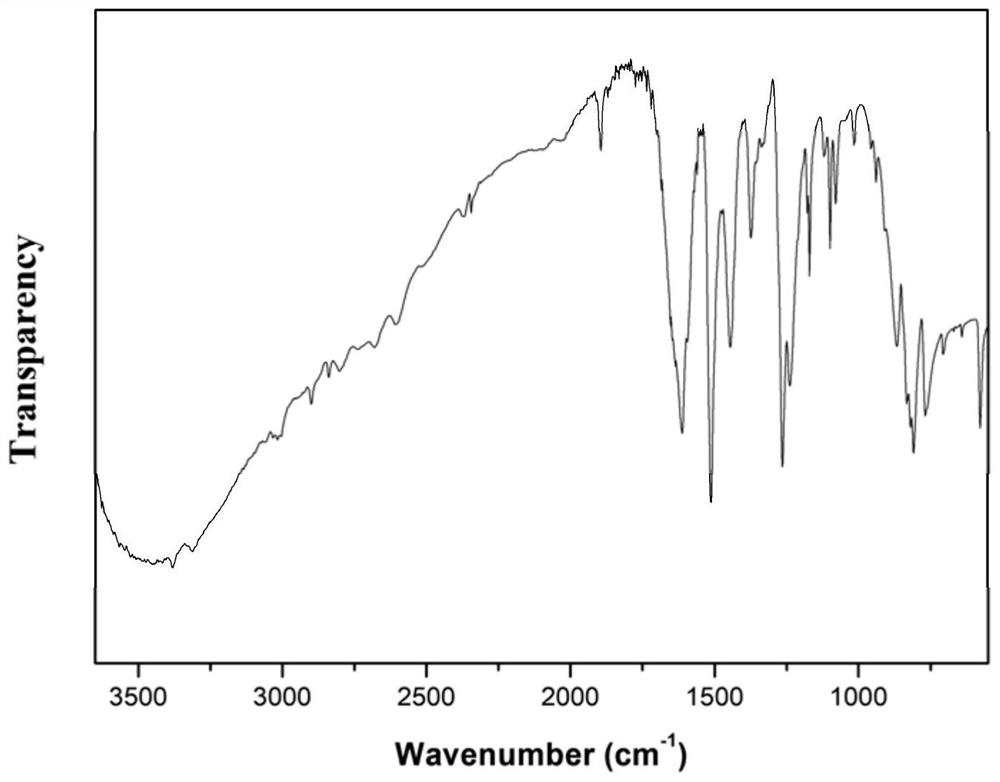
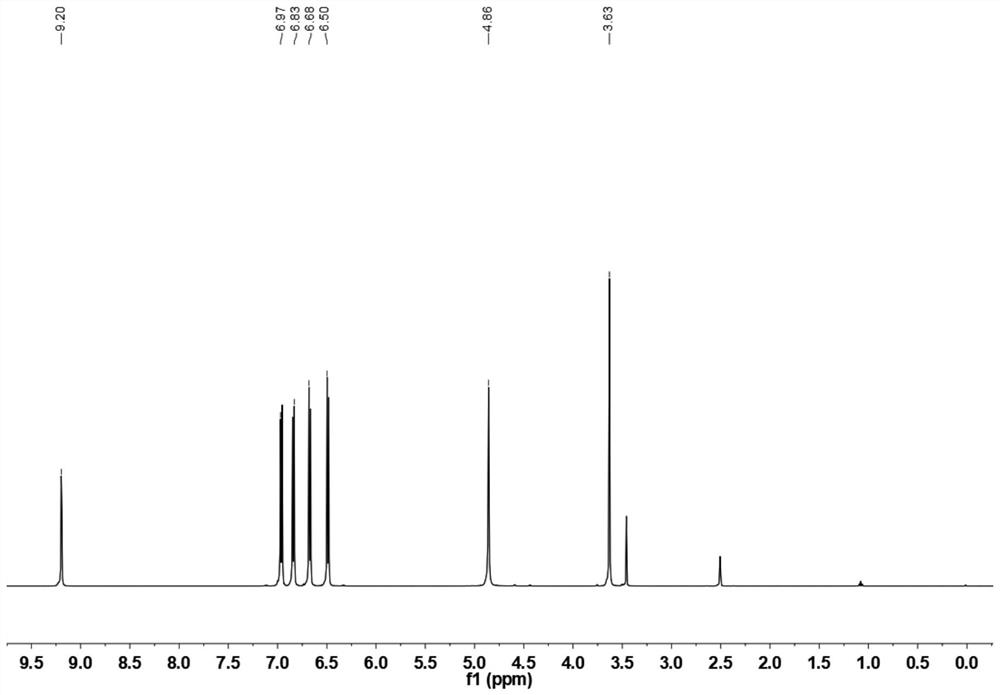
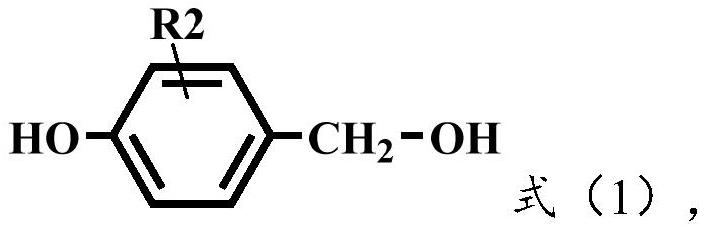
Preparation method of aminophenol containing methylene
A technology of aminophenol and methylene, applied in the field of aminophenol preparation, can solve the problems that the yield of aminophenol is difficult to exceed 65%, and the risk is high
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Embodiment 1: the preparation of 4-((4-aminophenyl)-methylene)phenol
[0052] 4-((4-aminophenyl)-methylene)phenol, the specific structural formula is shown in formula (4).
[0053]
[0054] The synthetic process of 4-((4-aminophenyl)-methylene)phenol shown in formula (4) is shown in the following formula:
[0055]
[0056] Specifically, the preparation method of 4-((4-aminophenyl)-methylene)phenol shown in formula (4) comprises the following steps:
[0057] Under a nitrogen gas atmosphere, mix aniline hydrochloride (26.0g, 0.2mol), p-hydroxymethylphenol (12.4g, 0.1mol), and aniline (24mL, 0.2mol) in a three-necked flask, heat the reaction, and heat the temperature of the reaction The temperature is 120°C, the heating reaction time is 1.5 hours, the mixture obtained after the heating reaction is poured into water, and NaHCO is added 3After neutralization to neutrality, add ethyl acetate for extraction and separation. After the organic layer is dried and the solve...
Embodiment 2
[0062] Compared with Example 1, the difference of Example 2 is only that the temperature of the heating reaction in Example 2 is 130° C., and the reaction time is 1 hour, and the obtained 4-((4-aminophenyl)-methylene ) The yield of phenol was 81.0%.
Embodiment 3
[0064] Compared with Example 1, the only difference in Example 3 is that the amount of aniline in Example 3 is 0.15mol, and the yield of 4-((4-aminophenyl)-methylene)phenol is 79.1 %.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com