A kind of preparation method of rucaparib intermediate of medicine for treating ovarian cancer
An intermediate, ovarian cancer technology, applied in the field of preparation of pharmaceutical intermediates, can solve the problems of long reaction time, complex process, harsh conditions, etc., and achieve the effects of shortening the reaction period, readily available raw materials, and mild and easily controllable reaction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] a) 6-fluoro-4-cyano-1H-indole
[0059]
[0060] The compound of formula I (460g), cuprous cyanide (290g) and N,N-dimethylformamide (2.3L) were mixed, replaced with nitrogen three times, heated to 150°C and stirred for 6 hours. After the reaction was completed, it was cooled to room temperature.
[0061] Water (7L) and ethyl acetate (2L) were added to the reaction solution, filtered through diatomaceous earth, the filtrate was extracted twice with ethyl acetate (2L*2), the organic layer was dried with anhydrous sodium sulfate, decolorized with activated carbon, Concentrate to dryness to obtain 310 g of gray solid (compound of formula II), purity: 97.51%, yield: 90%.
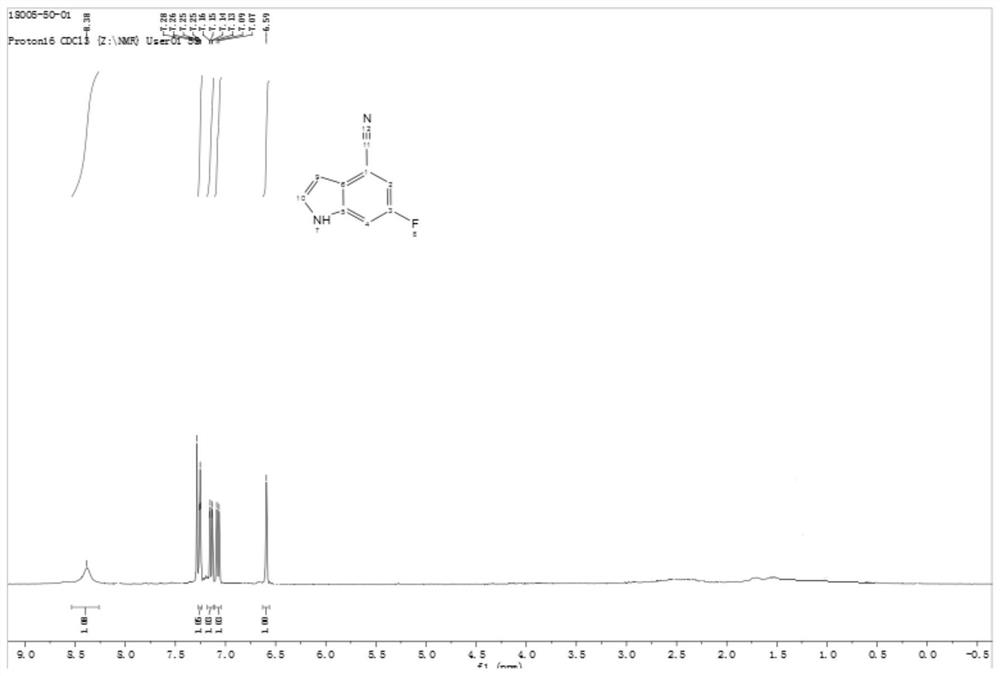
[0062] The HNMR spectrogram of formula II compound sees attached figure 1 .
[0063] HNMR (400MHz, CDCl 3 ): δ9.38(br s,1H),7.25(t,J=4.0Hz,1H),7.15(dd,J=8.0,4.0Hz,1H),7.08(d,J=8.0Hz,1H), 6.59 (t, J=4.0Hz, 1H).
[0064] b) 6-fluoro-4-carboxy-1H-indole
[0065]
[0066] Compound of formula II (29...
Embodiment 2
[0082] a) 6-fluoro-4-cyano-1H-indole
[0083]
[0084] Compound of formula I (360g), zinc cyanide (400g) and dimethylsulfoxide (1.8L) were mixed, and Pd(dppf)Cl was added 2 (18 g), nitrogen replacement 3 times. Heat to 120°C and stir for 8 hours. After the reaction was completed, it was cooled to room temperature.
[0085] Water (5.4L) and ethyl acetate (2L) were added to the reaction solution, filtered through diatomaceous earth, the filtrate was extracted twice with ethyl acetate (2L*2), the organic layer was dried with anhydrous sodium sulfate, decolorized with activated carbon, Concentrate to dryness to obtain 250 g of gray solid (compound of formula II), purity: 95.87%, yield: 93%.
[0086] b) 6-fluoro-4-carboxy-1H-indole
[0087]
[0088] The compound of formula II (240 g) and concentrated hydrochloric acid (1.2 L) were added into a pressure reactor, heated to 120° C. and stirred for 10 hours. After the reaction was completed, it was cooled to room temperature...
Embodiment 3
[0098] a) 6-fluoro-4-cyano-1H-indole
[0099]
[0100] The compound of formula I (750g), cuprous cyanide (377g), potassium iodide (872g) and N,N'-dimethylformamide (3.7L) were mixed, and replaced with nitrogen three times. Heat to reflux and stir for 16 hours. Cool to room temperature.
[0101] Add water (11L) and ethyl acetate (3L) to the reaction kettle, filter through diatomaceous earth, extract the filtrate twice with ethyl acetate (3L*2), dry the organic layer with anhydrous sodium sulfate, decolorize with activated carbon, Concentrate to dryness to obtain 455 g of gray solid (compound of formula II), purity: 98.01%, yield: 81%.
[0102] b) 6-fluoro-4-carboxy-1H-indole
[0103]
[0104] Compound of formula II (510 g), potassium hydroxide (893 g), ethylene glycol (1.2 L) and 2.4 L of water were mixed, heated to 120° C. and stirred for 20 hours. After the reaction was completed, it was cooled to room temperature.
[0105] Water (7 L) was added to the reaction ket...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com