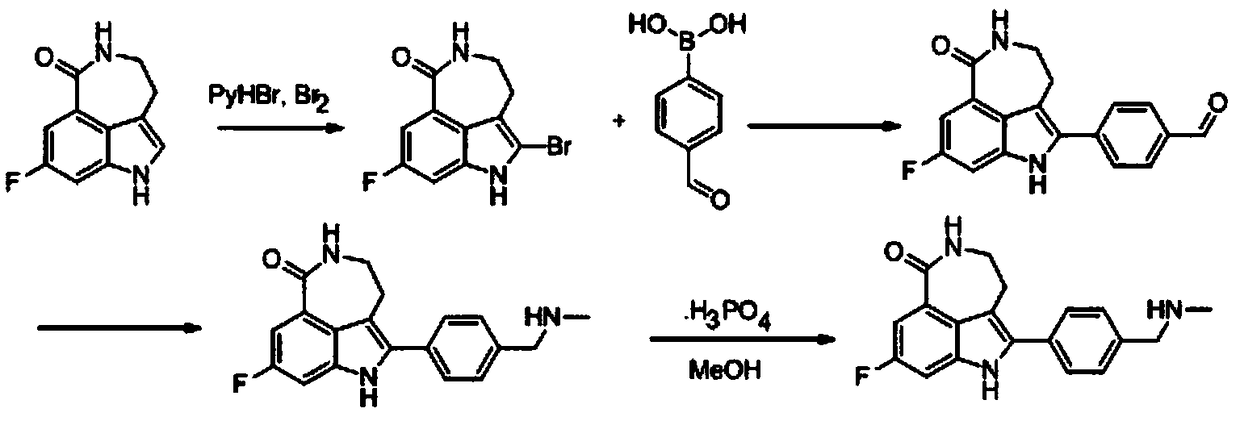
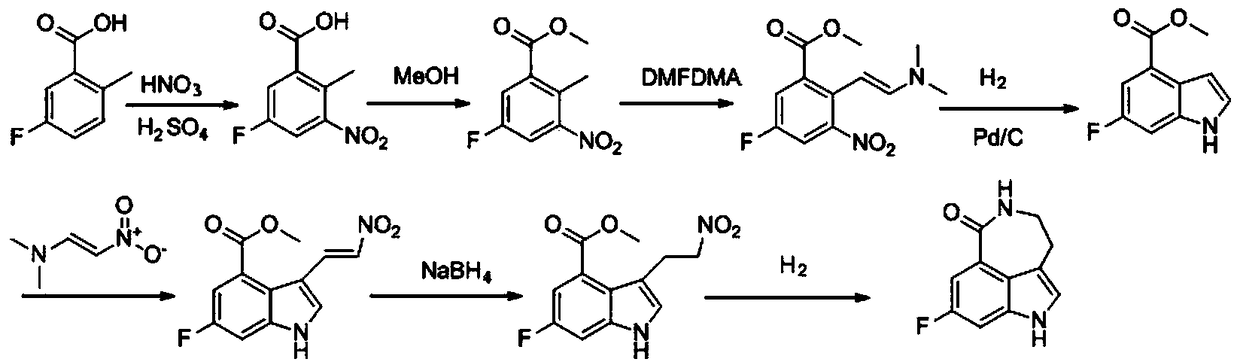
A kind of preparation method of key intermediate of anti-ovarian cancer drug rucaparib
An intermediate and ovarian cancer technology, which is applied in the field of preparation of pharmaceutical intermediates, can solve problems such as potential safety hazards and environmental pollution, unfavorable safety production, and scarce raw material costs, so as to ensure safe production, facilitate industrial production, and avoid explosion risks. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
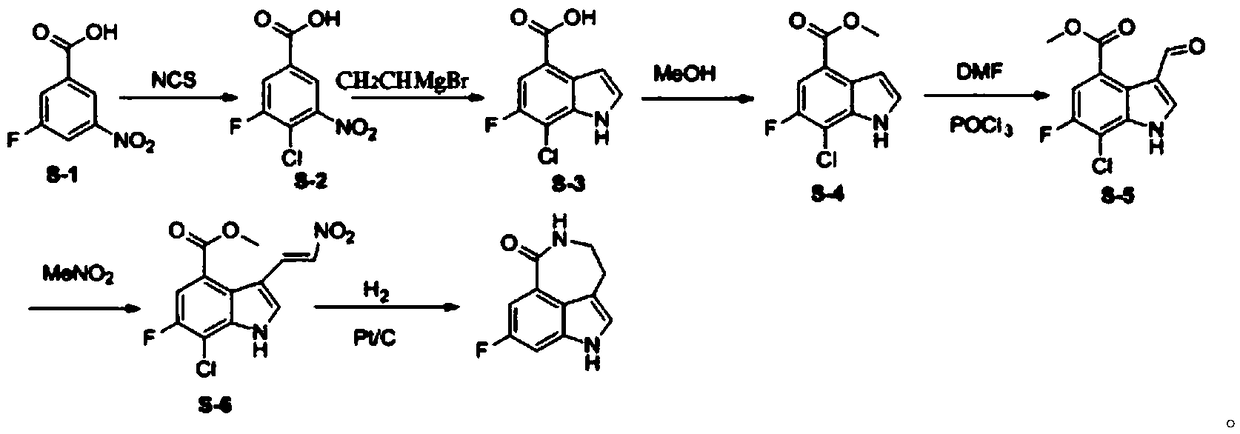
[0039] Step 1: Dissolve compound S-1 (18.5g, 0.1mol) in 150ml of N,N-dimethylformamide, add NCS (N-chlorosuccinimide) (13.3g, 0.1mol) , the reaction solution was reacted at 35°C for 4 hours, and then the insoluble matter was filtered, extracted with ethyl acetate, and washed with water. The organic solvent was dried with anhydrous sodium sulfate, and spin-dried to obtain 20 g of compound S-2, which was directly used in the next reaction.
[0040] Step 2: Dissolve compound S-2 (20g, 0.09mol) in tetrahydrofuran, add vinylmagnesium bromide (1M, 360mL) dropwise at -30°C, continue to react for 1 hour after the dropwise addition, and chlorinate with saturated The reaction was quenched with ammonium, extracted with ethyl acetate, and the organic layer was separated. The organic phase was spin-dried and recrystallized from ethyl acetate to obtain 8 g of pure compound S-3.
[0041] Step 3: Dissolve compound S-3 (16g, 0.083mol) in 150ml of methanol, add thionyl chloride (4.8mL, 0.066m...
Embodiment 2
[0046] Step 1: Dissolve compound S-1 (18.5g, 0.1mol) in 150ml of N,N-dimethylformamide, add NCS (N-chlorosuccinimide) (19.9g, 0.15mol) , the reaction solution was reacted at 45°C for 10 hours, and then the insoluble matter was filtered, extracted with ethyl acetate, and washed with water. The organic solvent was dried with anhydrous sodium sulfate, and spin-dried to obtain 20.6 g of compound S-2, which was directly used in the next reaction.
[0047] Step 2: Dissolve compound S-2 (20g, 0.09mol) in tetrahydrofuran, add vinylmagnesium bromide (1M, 180mL) dropwise at -45°C, continue the reaction for 1.5 hours after the dropwise addition, and then dissolve the mixture with saturated ammonium chloride The reaction was quenched, extracted with ethyl acetate, and the organic layer was separated. The organic phase was spin-dried and recrystallized from ethyl acetate to obtain 9.5 g of pure compound S-3.
[0048]Step 3: Compound S-3 (16 g, 0.083 mol) was dissolved in 150 ml of methan...
Embodiment 3
[0053] Step 1: Dissolve compound S-1 (18.5g, 0.1mol) in 150ml of N,N-dimethylformamide, add NCS (N-chlorosuccinimide) (16g, 0.12mol), The reaction solution was reacted at 50°C for 24 hours, and then the insoluble matter was filtered, extracted with ethyl acetate, and washed with water. The organic solvent was dried with anhydrous sodium sulfate, and spin-dried to obtain 18.5 g of compound S-2, which was directly used in the next reaction.
[0054] Step 2: Dissolve compound S-2 (20g, 0.09mol) in tetrahydrofuran, add vinylmagnesium bromide (1M, 270mL) dropwise at -10°C, continue to react for 2 hours after the dropwise addition, and dissolve with saturated ammonium chloride The reaction was quenched, extracted with ethyl acetate, and the organic layer was separated. The organic phase was spin-dried and recrystallized from ethyl acetate to obtain 10 g of pure compound S-3.
[0055] Step 3: Compound S-3 (16 g, 0.083 mol) was dissolved in 150 ml of methanol, and thionyl chloride (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com