Near-infrared fluorescent molecule with double (multi) photon excitation and synthesis method of near-infrared fluorescent molecule
A compound, selected technology, applied in material excitation analysis, fluorescence/phosphorescence, chemical instruments and methods, etc., can solve the problems of limited spatial resolution, poor soft tissue contrast, etc., achieve small scattering coefficient, strong penetrating ability, low The effect of autofluorescence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0112] This embodiment synthesizes compound 1 according to the following route:
[0113]
[0114] (1) Synthesis of compound 5a
[0115] 4-(diphenylamino)phenylboronic acid, 4,7-dibromo-5,6-dinitro-2,1,3-benzothiadiazole, potassium carbonate, tetrakis(triphenylphosphine) Add dioxane and water to palladium in a molar ratio of 1:2.5:10:0.04, the volume ratio of dioxane and water is 4:1, under the protection of an inert gas, heat and reflux for 12h, and purify to obtain compound 5a, producing rate 56%.
[0116] (2) Synthesis of compound 6a
[0117] Compound 5a and iron powder were dissolved in acetic acid at a molar ratio of 1:12. The amount of acetic acid added was sufficient to dissolve the raw materials. The reaction was carried out at 100 degrees Celsius for 5 hours for purification to obtain compound 6a with a yield of 93%.
[0118] (3) Synthesis of compound 1
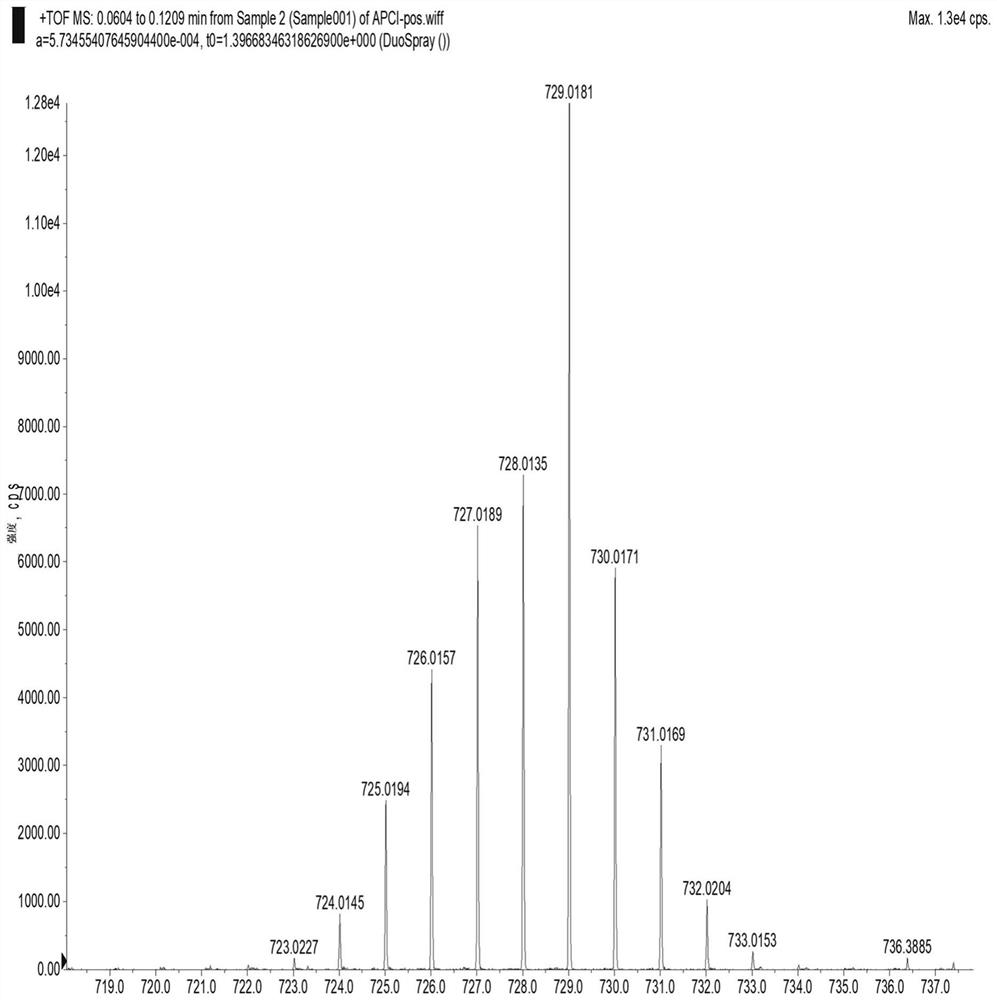
[0119] Dissolve compound 6a and selenium dioxide in dichloromethane at a molar ratio of 1:2.5, the amount ...
Embodiment 2
[0123] This embodiment synthesizes compound 31 according to the following route:
[0124]
[0125] (1) Synthesis of compound 1
[0126] Compound 6a and benzil were dissolved in acetic acid and chloroform at a molar ratio of 1:1.2, the solvent volume ratio was 1:1, and purified at 60°C for 8 hours to obtain compound 31 with a yield of 90%.
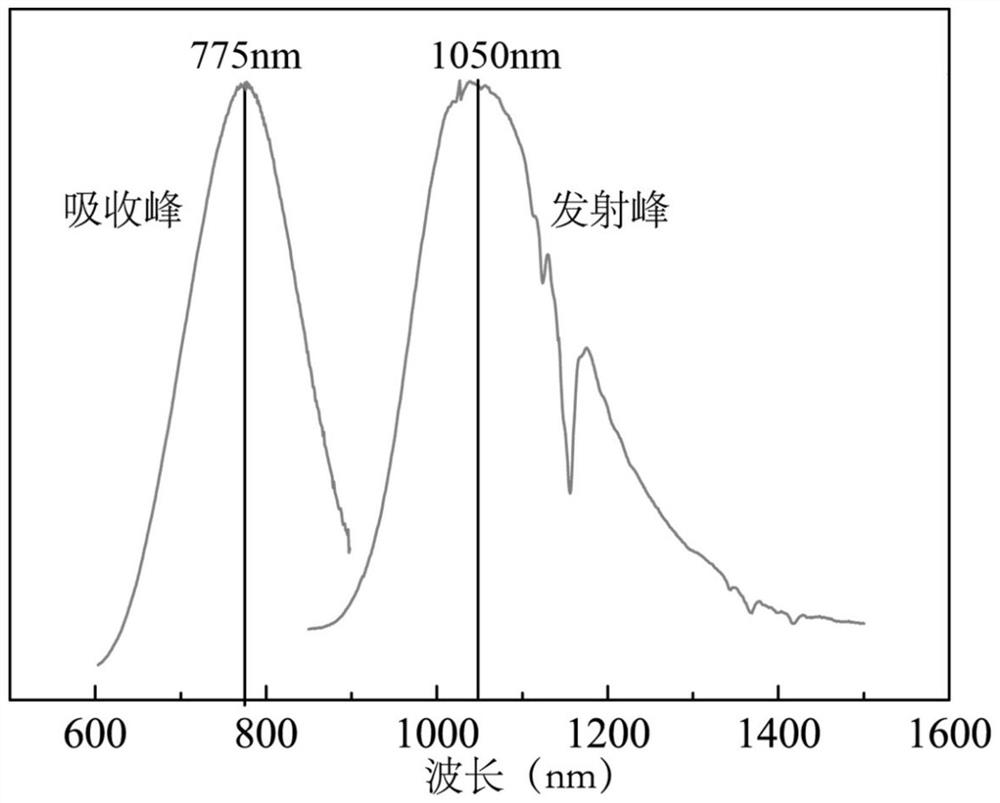
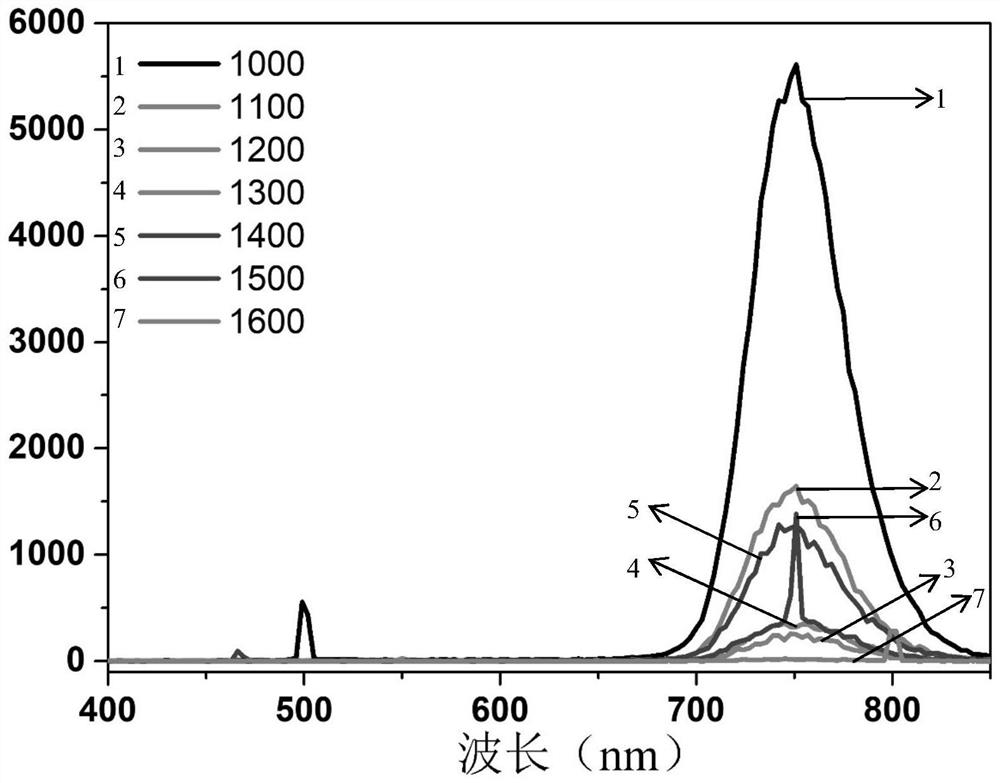
[0127] image 3 It is the emission spectrum of compound 31 under two-photon excitation. In the figure, curve 1 represents that the emission wavelength is about 500nm and 750nm when the excitation wavelength is 1000nm; curve 2 represents that the emission wavelength is about 750nm when the excitation wavelength is 1100nm; curve 3 represents that the emission wavelength is about 750nm when the excitation wavelength is 1200nm Its emission wavelength is about 750nm when curve 4 represents excitation wavelength is 1300nm; Its emission wavelength is approximately 750nm when curve 5 represents excitation wavelength is 1400nm; Its emission wav...
Embodiment 3
[0129] This embodiment synthesizes compound 45 according to the following route:
[0130]
[0131] (1) Synthesis of Compound 43
[0132] Add compound 41, compound 42, potassium carbonate, tetrakis(triphenylphosphine) palladium in a molar ratio of 1:5:20:0.08 to dioxane and water, and the volume ratio of dioxane to water is 8:1, Under the protection of nitrogen, it was heated to reflux for 24 h for purification to obtain compound 43.
[0133] (2) Synthesis of compound 44
[0134] Dissolve compound 43 and iron powder in acetic acid at a molar ratio of 1:12, the amount of acetic acid added can dissolve the raw materials, react at 100 degrees Celsius for 12 hours for purification, and compound 44 is obtained.
[0135] (3) Synthesis of compound 45
[0136] Dissolve compound 44 and selenium dioxide in dichloromethane at a molar ratio of 1:4, the amount of dichloromethane can dissolve the raw material, react at room temperature for 8 hours for purification, and obtain compound ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com