IL-6-IL-27 compound and application thereof in preparation of antiviral drugs
An antiviral drug, IL-27 technology, applied in the field of biomedical detection, to achieve the effect of inhibition of replication level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
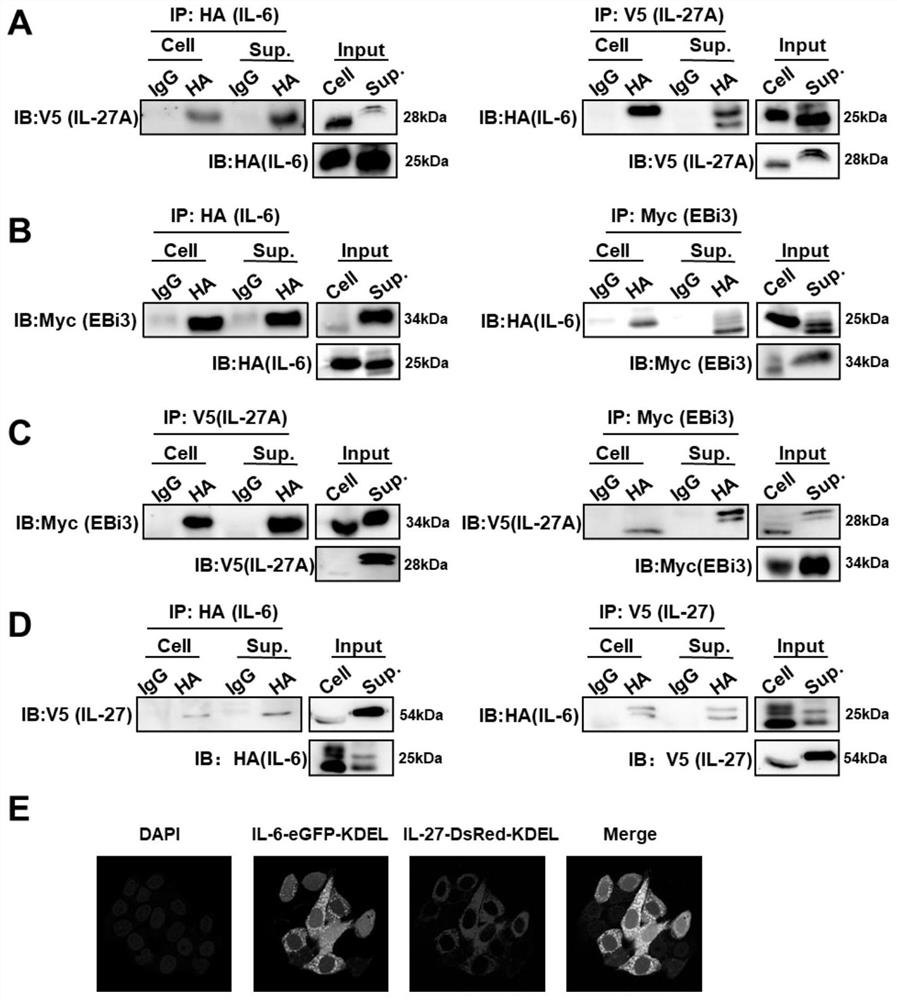
[0059] The construction of embodiment 1 expression vector
[0060] All expression plasmids in this patent are constructed on the pcDNA3.1(+) vector, and cloned using the homologous recombination cloning kit of Qingdao Biotechnology Co., Ltd. Total cellular RNA was extracted from SeV-infected human THP1 cells, and the cDNAs of human IL-6, EBi3 and IL-27A were amplified and cloned directly into the pcDNA3.1(+) vector. IL-6 was tagged with C-terminal 3×HA, EBi3 was tagged with C-terminal Myc, and IL-27A was tagged with C-terminal V5. In the IL-27 expression plasmid, the coding sequences of EBi3 and IL27A were linked with the sequence GSGSGGSGGSGSGKL coding the peptide chain, and a C-terminal V5 tag was added. The IL-6 fluorescent protein consists of IL6, GFP and the C-terminal ER retention signal KDEL, named IL6-GFP-KDEL. The IL-27 fluorescent protein consists of IL27, DsRed and a C-terminal KDEL signal, named IL27-DsRed-KDEL.
[0061] In addition, plasmids overexpressing MyD8...
Embodiment 2
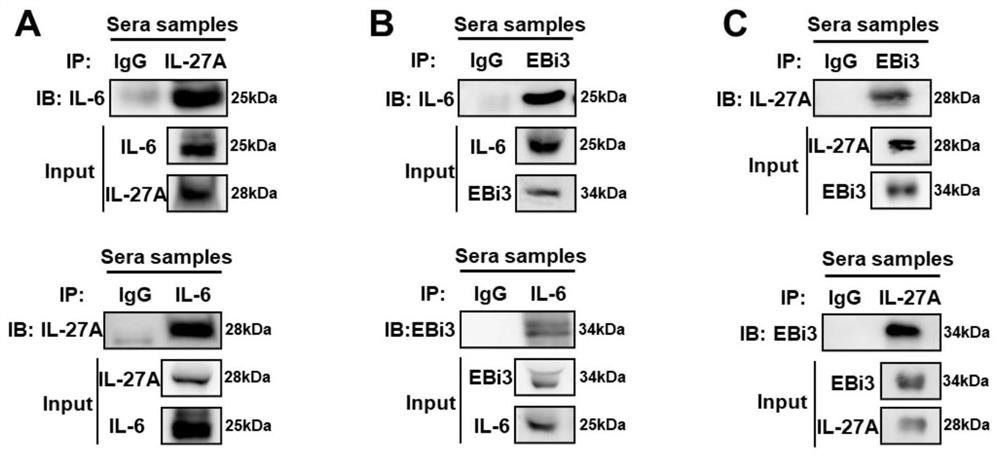
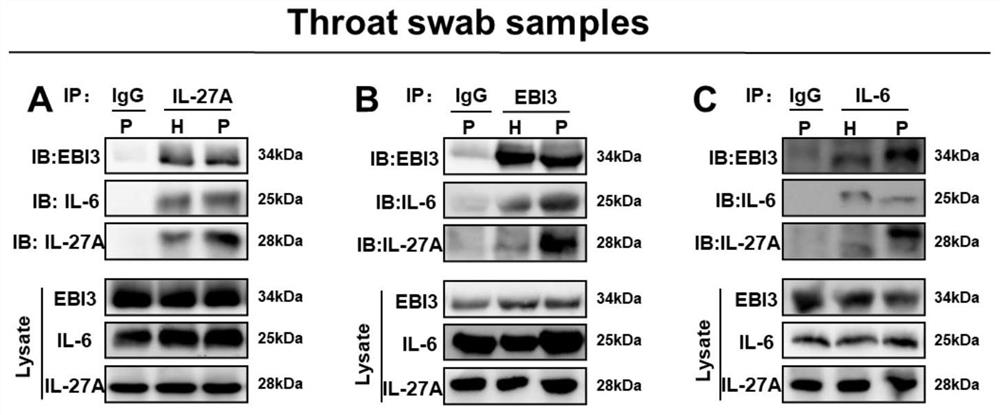
[0086] Embodiment 2, endogenous interaction
[0087] 1. Serum samples
[0088] The serum samples of 10 patients with influenza virus infection and the throat swab samples of 45 patients with influenza virus infection used in this patent are all from Hubei Provincial Center for Disease Control and Prevention. Collection of clinical samples was performed in accordance with the principles of the Declaration of Helsinki and was approved by the Institutional Review Board of Wuhan University in accordance with the Guidelines for the Protection of Human Subjects. All study participants provided written informed consent for sample collection and subsequent analysis.
[0089] 2. Processing of serum samples
[0090] Serum samples from 10 IAV-infected patients were obtained from Hubei Provincial Center for Disease Control and Prevention. Serum samples were mixed and divided into three groups, and 1ml samples were taken from each group for CoIP experiments. The rest of the samples wer...
Embodiment 3
[0096] Example 3 Determination of interactions by co-immunoprecipitation
[0097] The IL-6-HA, IL-27-V5, IL-27A-V5 and EBi3-Myc expression plasmids constructed in Example 1 were co-transformed in different combinations. After 30 hours of co-transformation, the cells were harvested and an appropriate amount of cells were added for lysis Buffer solution (containing protease inhibitors), lysed on ice for 30 minutes, and the cell lysate was centrifuged at the maximum speed for 15 minutes at 4°C, and the supernatant was taken to obtain the total cell protein. Among them, the KD of IL-6-HA, IL-27-V5, IL-27A-V5 and EBi3-Myc proteins are 25KD, 28KD, 54KD and 34KD respectively;
[0098] Take a small amount of lysate for Western blot analysis (input analysis);
[0099] Add 1 μg of the corresponding antibody to the remaining lysate (the remaining lysate is divided into 4 parts, one part is incubated with 1ug anti-HA antibody, one part is incubated with 1ug IgG, one part is incubated wit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com