Pig samhd1 gene, protein, monoclonal antibody and application thereof
A technology of monoclonal antibody and gene, applied in application, genetic engineering, plant gene improvement, etc., can solve the problems of poor protein reactivity, no reactivity, no accurate complete gene sequence of porcine SAMHD1, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 Using the RACE method to amplify and obtain the whole gene sequence of porcine SAMHD1
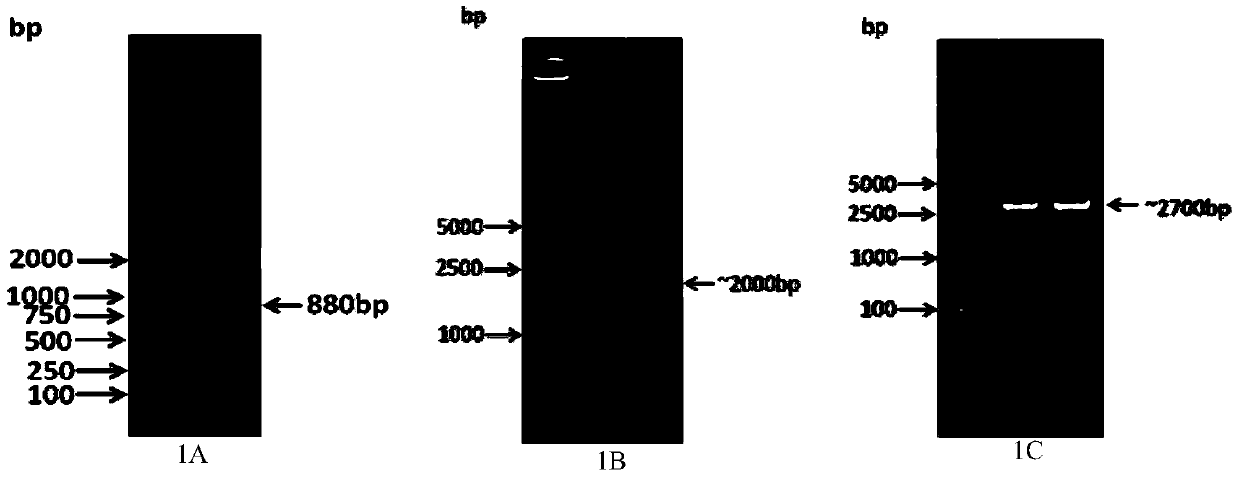
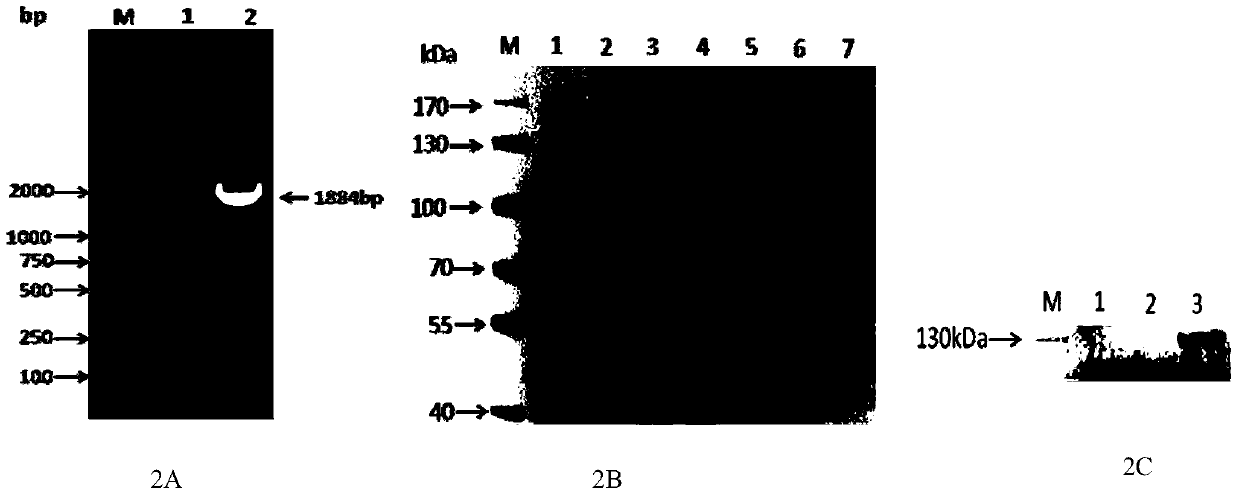
[0032] By comparing the predicted sequence of porcine SAMHD1 submitted in GenBank (Genbank ID: XM_003483952, XM_003361435, AK236802), a pair of primers were designed to amplify the partial nucleic acid sequence of SAMHD1. The sequence of the pair of primers was s880-forward: 5'-TCAAAGCAGACCCTTACAT-3'( SEQ ID NO. 3), S880-reverse: 5'-TTGCCTAATTCCTGTTTCT-3' (SEQ ID NO. 4). As a result, a partial gene sequence of pig SAMHD1 with a length of about 880bp was amplified (see figure 1A). After the sequence was sequenced and compared correctly, a pair of SAMHD1 gene-specific primers were designed according to the amplified 880bp sequence, GSP1-forward: 5'-AGATGCCTTCCTCAAAGCAGACCCT-3' (SEQ ID NO.5), GSP1-reverse: 5'-TATGCCCTAAATTGATTGGGGGGGC -3'(SEQ ID NO.6), and a pair of inner nested primers about 220bp in length are designed inside it, S220-forward: 5'-GCTGAACTGAAGGCTGAA GAT-3'(...
Embodiment 2
[0033] Example 2 Recombinant expression of porcine SAMHD1 protein
[0034] According to the pig SAMHD1 complete gene sequence amplified in Example 1, specific primers are designed to amplify the SAMHD1 coding sequence, and the specific primers for amplifying the coding gene are as follows, pcold-pig SAMHD1 (pcold-pig SAMHD1-forward: 5'- CC AAGCTT ATGCAGAGTGCCGACTCC-3' (SEQ ID NO.9), pcold-pig SAMHD1-reverse: 5'-CG GGATCC TCACACCGAGTCCTTTGCA-3'(SEQ ID NO.10)), wherein the upstream and downstream primers contain Hind III and Bam HI restriction endonuclease sites respectively, and the SAMHD1 coding sequence amplified by pcold-pig SAMHD1 primers is used to insert pcold TF DNA prokaryotic expression vector; pFLAG-pig SAMHD1 (pFLAG-pig SAMHD1 forward: C AAGCTT ATGCAGAGTGCCGACTCCCAGCAG (SEQ ID NO. 11); pFLAG-pig SAMHD1 reverse: GC TCTAGA TCACACCGAGTCCTTTGCAAA (SEQ ID NO.12)), the SAMHD1 coding sequence amplified by the pFLAG-pig SAMHD1 primer, used to insert into the p3×FLAG C...
Embodiment 3
[0037] Example 3 Preparation of porcine SAMHD1 mouse monoclonal antibody
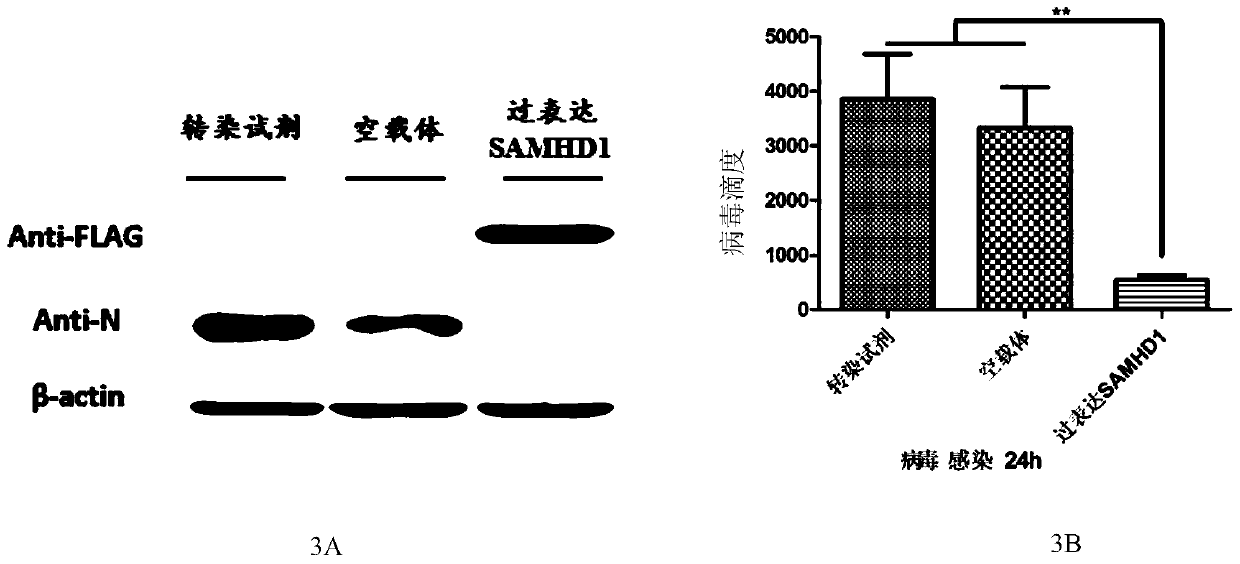
[0038] The prokaryotically expressed and purified SAMHD1 protein in Example 2 was used as an immunogen to immunize mice for the preparation of monoclonal antibodies. Eight-week-old BABL / c female mice were selected, and the purified porcine SAMHD1 protein was immunized with prokaryotic expression. The dose of immunization is 100 micrograms of protein for the first time, and 50 micrograms for the second to fourth times, respectively, with a total volume of 100 ul, injected subcutaneously at multiple points, with an interval of two weeks. The first immunization was emulsified with Freund's complete adjuvant, SAMHD1 protein was mixed with Freund's complete adjuvant at a ratio of 1:1, and the second immunization was emulsified with Freund's incomplete adjuvant, SAMHD1 protein was mixed with Freund's incomplete adjuvant 1: Mixed at a ratio of 1, without adding adjuvant for the third and fourth immunizations,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com