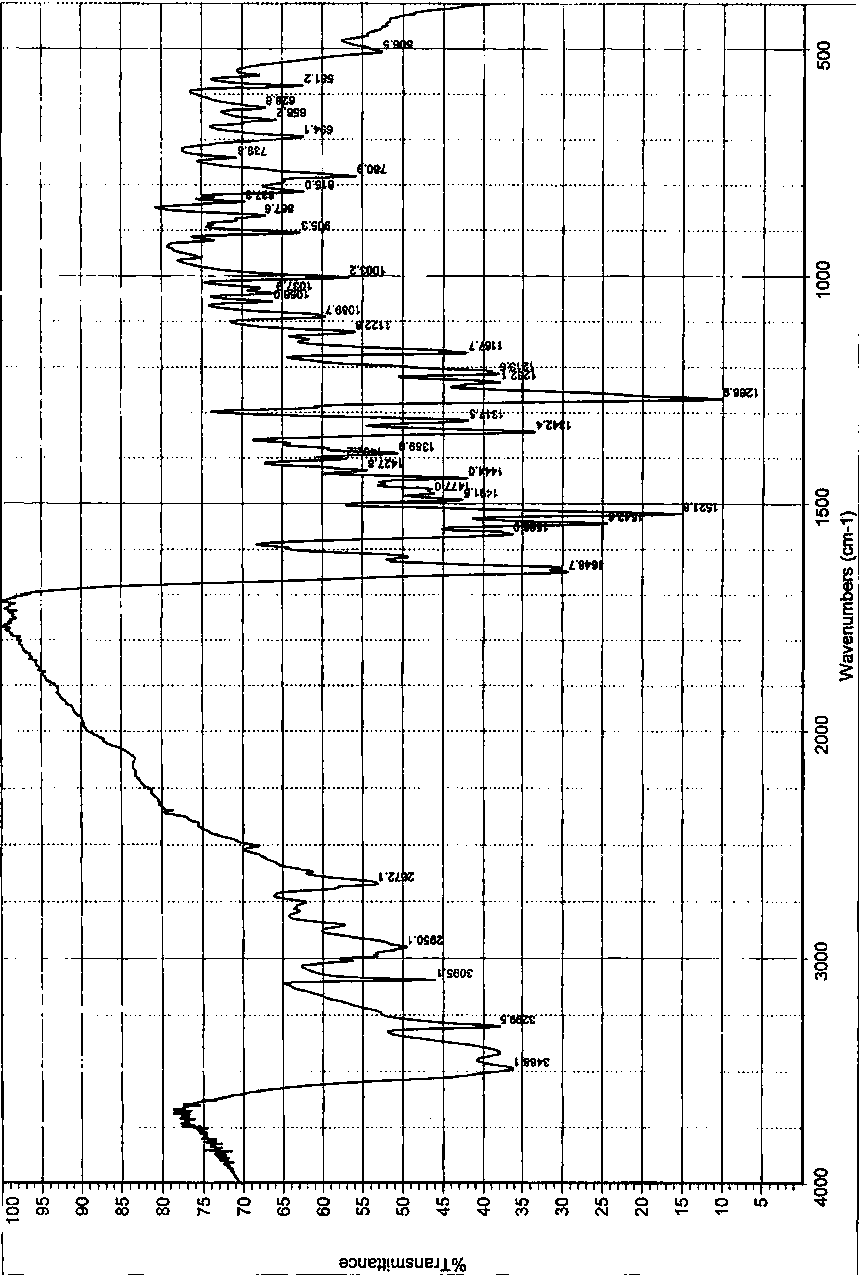
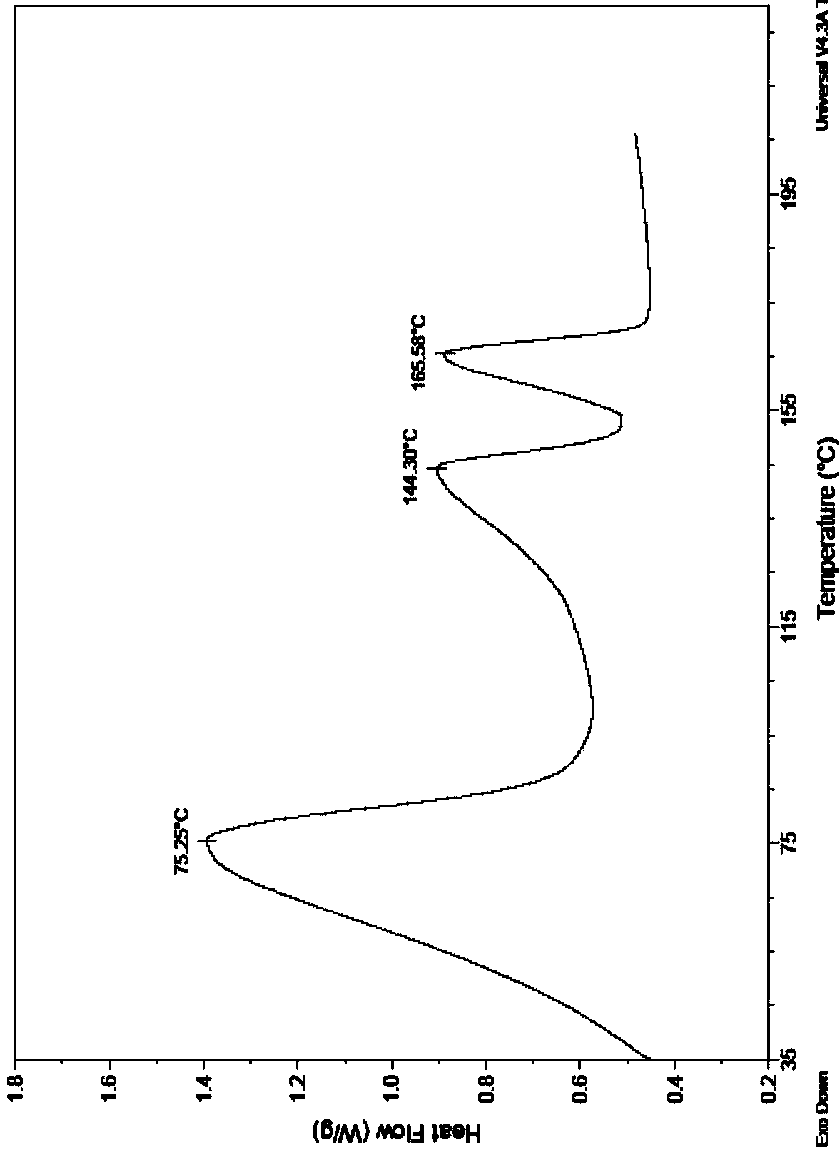
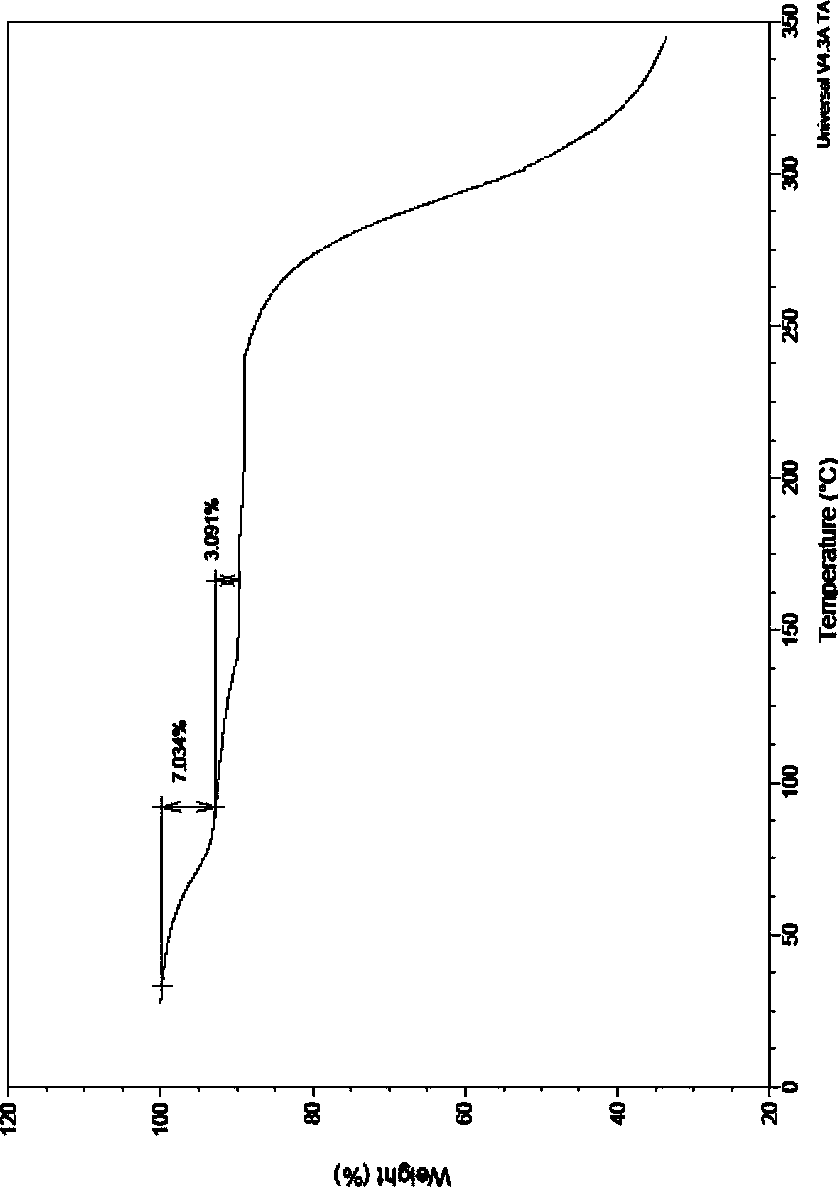
Acotiamide hydrochloride hydrate crystal form and preparation method thereof
A technology of acotiamide hydrochloride trihydrate and acotiamide hydrochloride, which is applied in the field of medicine, can solve problems such as affecting drug efficacy and differences, and achieves the effects of good repeatability, single crystal form and stable properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Take 3.0 g of acotiamide hydrochloride hydrate and put it into 12.0 g of 20% aqueous methanol solution, and heat to reflux until dissolved. Cool to 20°C and filter. The filter cake was air-dried at 50°C until the moisture content was <8%. Place it in the air at room temperature until the water content is 9.8%-10.5%, to obtain 2.31 g of type A crystalline acotiamide hydrochloride hydrate with a yield of 77.0%.
Embodiment 2
[0036] Take 2.0 g of acotiamide hydrochloride anhydrate and put it into 8.0 g of 40% isopropanol aqueous solution, and heat to reflux until dissolved. Cool to 20°C and filter. The filter cake was air-dried at 50°C until the moisture content was <8%. Place in the air at room temperature until the water content is 9.8%-10.5%, to obtain 2.0 g of type A crystalline acotiamide hydrochloride hydrate with a yield of 90.0%.
Embodiment 3
[0038] Take 3.0 g of acotiamide hydrochloride hydrate and put it into 12.0 g of 20% acetonitrile aqueous solution, and heat to reflux until dissolved. Cool to 20°C and filter. The filter cake was air-dried at 50°C until the moisture content was <8%. Place in the air at room temperature until the water content is 9.8%-10.5%, to obtain 2.59 g of type A crystalline acotiamide hydrochloride hydrate with a yield of 86.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com