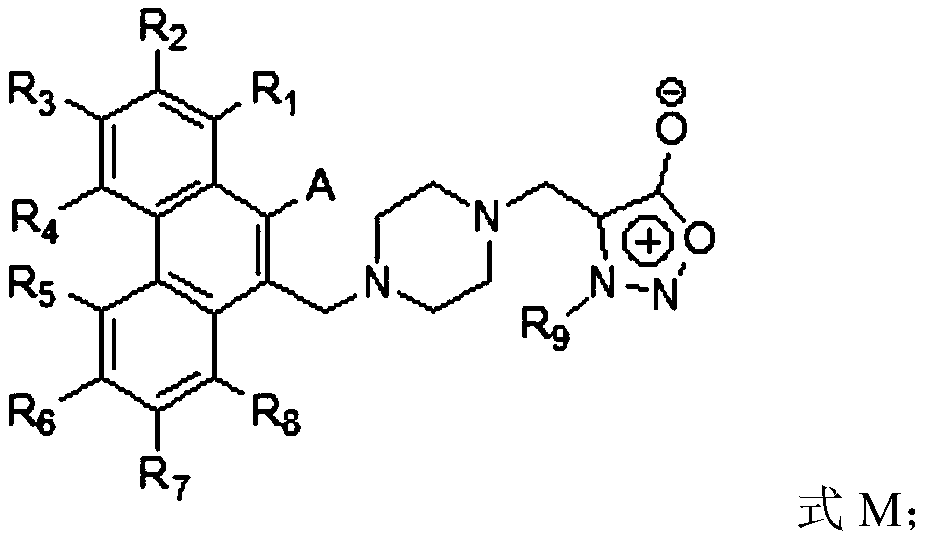
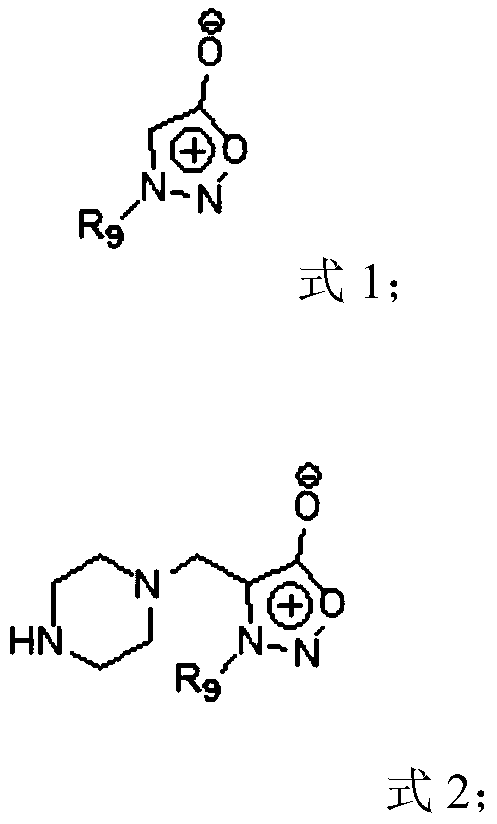
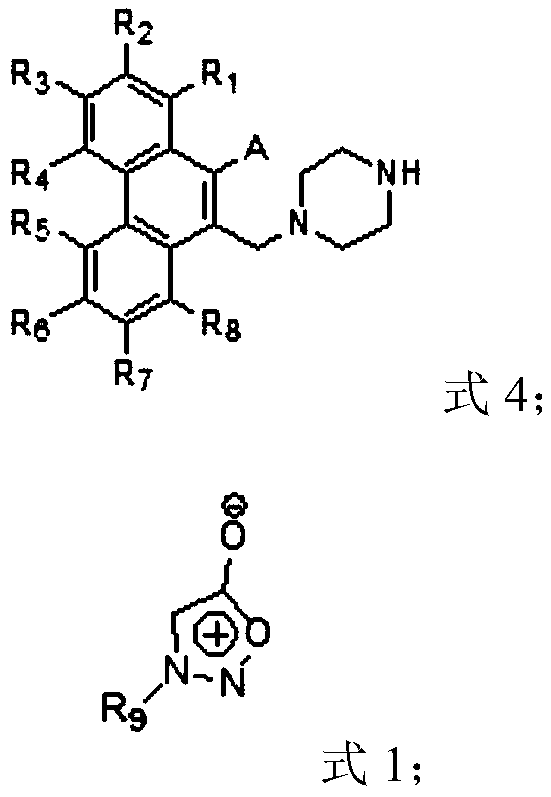
Compound, and preparation method and application thereof
A compound and low-level technology, applied in organic chemistry, drug combination, pharmaceutical formula, etc., can solve the problems of anti-cancer effect gap, ataxia, toxicity, etc., and achieve significant anti-cancer effect and good anti-cancer effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0106] Embodiment 1: the preparation of compound MA1
[0107]
[0108] 1.1 Preparation of compound 2A:
[0109] Add ethanol (10mL), compound 1A (1g), piperazine (1.06), and paraformaldehyde (0.37g) into the reaction flask in turn, add concentrated hydrochloric acid (0.5mL) while stirring, heat to 70°C, and stir until compound 1 After the reaction is complete, most of the ethanol is concentrated under reduced pressure, the solid is precipitated, filtered with suction, the solid is washed with methyl tert-butyl ether, the solid is dissolved in water, the pH value is adjusted to ≈8 by adding saturated sodium bicarbonate, and the extracted with base ether, combined the organic layers, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain compound 2A (1.12 g), which was directly cast down. LCMS(ESI): 261.1[M+H] + .
[0110] Add methanol (10mL), compound 1A (1g), piperazine (1.06), and paraformaldehyde (0.74g) into the reaction flask in sequenc...
Embodiment 2
[0121] Embodiment 2: the preparation of compound MB1
[0122]
[0123] 2.1 Preparation of Compound 4B:
[0124] When B is Br:
[0125] Add acetonitrile (100mL), compound 3B (10g), and piperazine (3.58g) into the reaction flask in sequence, heat to 80°C, stir until compound 3B reacts completely, cool down to room temperature, add saturated brine to wash, wash with anhydrous sulfuric acid Dry over sodium and concentrate under reduced pressure to obtain compound 4B (9.5 g), which is directly cast down. LCMS(ESI): 367.1[M+H] + . .
[0126]Add N,N-dimethylformamide (100mL), compound 3B (10g), piperazine (4.77g), and potassium carbonate (3.83g) into the reaction flask in sequence, heat to 70°C, and stir until the reaction of compound 3B is complete , filtered, washed with ethyl acetate, washed with saturated brine, the aqueous phase was extracted with ethyl acetate, the organic phases were combined, dried with anhydrous sodium sulfate, and concentrated under reduced pressure...
experiment example 1
[0183] Experimental example 1: Determination of anti-tumor activity in vitro (MTT method)
[0184] In order to measure the in vitro antitumor activity of the compounds of the present invention, the compounds prepared in the examples of the present invention were determined, and the experimental steps are:
[0185] 1. Culture normal growth tumor cells with 1×10 4 The cells / mL were inoculated into a 96-well plate (100 μL per well), and incubated at 37° C. in a 5% CO2 incubator for 24 hours.
[0186] 2. Add the test compound respectively, and cultivate for 5 days in a 5% CO2, complete humidity incubator.
[0187] 3. Discard the culture medium, add 100 μL of 0.04% MTT to each well, and incubate under the same conditions for 4 hours.
[0188] 4. Abandon the culture medium, add DMSO (150 μL per well), mix and record the light absorbance colorimetrically at a measurement wavelength of 570 nm and a reference wavelength of 450 nm, and calculate the inhibitory rate of the compound on ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com