Heparin molecule containing AT binding sequence and continuous 2-O-glucuronic acid residues, preparation method and application thereof
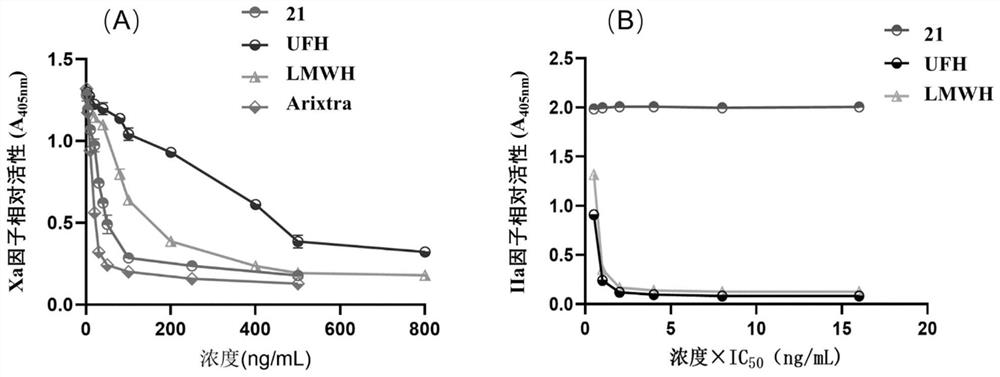
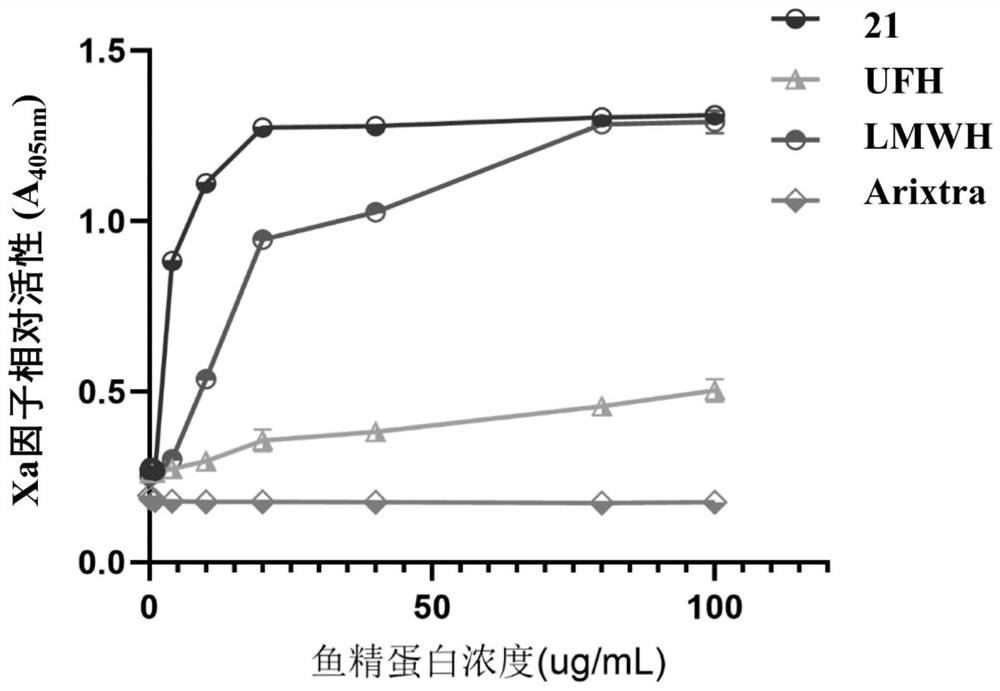
A technology of residues and heparin, which can be applied in drug combinations, extracellular fluid diseases, blood diseases, etc., can solve the problems of poor pharmacokinetics of compounds, catalytic modification efficiency lower than that of IdoA residues, unrealistic problems, etc., to achieve good results Industrial application prospects, strong and specific anti-FXa activity, and the effect of high-quality anticoagulant and antithrombotic drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
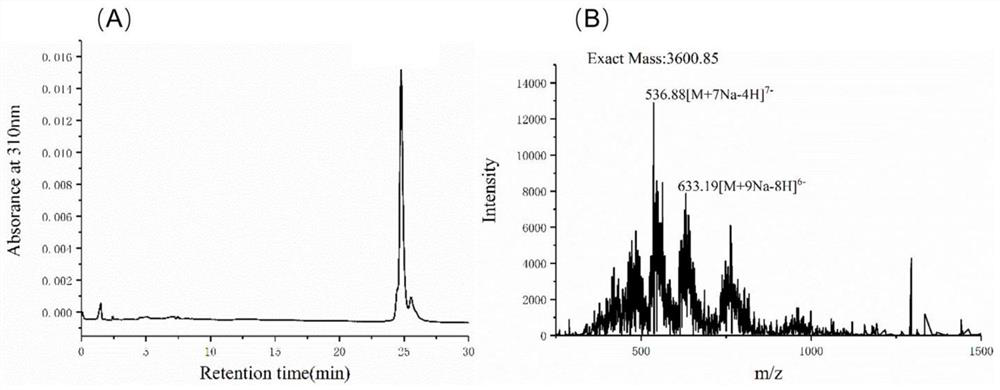
[0079] Example 1: Preparation of rare heparin pentasaccharide 5 containing 2 consecutive GlcA2S
[0080] Weigh 500mg of nitrophenyl-β-D-glucuronide (GlcA-PNP, 1) and dissolve it in ~200mL 50mmol / LTris-HCl buffer (containing 6mmol / L MnCl 2 , pH=7.5), while adding 1.2 times the equivalent of UDP-GlcNTFA and 5mL KfiA enzyme, stirring at room temperature overnight, the reaction was detected by PAMN-HPLC, and the chromatographic conditions were 0→100% KH within 45min 2 PO 4 Gradient elution, the flow rate is 0.5mL / min, and the detection wavelength is 310nm. When the yield is ≥95%, adjust the pH to 2-3 with trifluoroacetic acid (TFA) to stop the reaction, and the reaction solution is purified with a C18 chromatography column (3.0×50 cm), eluting with methanol-water containing 0.1% TFA, and the yield is to the target component. The obtained disaccharide skeleton was placed in 200 mL of the same buffer as above, while 1.2 equivalents of UDP-GlcA and 5 mL of PmHS2 enzyme were added,...
Embodiment 2
[0085] Example 2: Preparation of rare heptasaccharide 7 containing 3 consecutive GlcA2S
[0086] Take ~200mg of pentasaccharide skeleton 3, and refer to Example 1 to carry out enzymatic sugar chain extension by KfiA and PmHS2 in sequence to obtain heptasaccharide skeleton 6, whose molecular weight as measured by ESI-MS is consistent with the theoretical value; then LiOH is used to remove trifluoroacetyl, NST catalyzed N-sulfation modification to obtain heptasaccharide 7, the purity of which was >99% as measured by PAMN-HPLC, and the molecular weight as measured by ESI-MS was 1566.32Da.
[0087] The obtained heptasaccharide 7 was modified by 2OST enzymatically with reference to Example 1, the amount of PAPS added was changed to 4 times, and the reaction solution was purified to obtain product 8, whose purity was >99% as measured by PAMN-HPLC. ESI-MS measured that its component was 1805.92Da, and compared with substrate 7, three sulfuric acid groups were added. by 7 1 H-NMR (6...
Embodiment 3
[0090] Example 3: Preparation of rare heparin nonasaccharide 13 containing 4 consecutive GlcA2S
[0091] Take 100 mg of rare heparin pentasaccharide 5 containing 2 GlcA2S residues, carry out enzymatic sugar chain extension by KfiA and PmHS2 to form heparin nonasaccharide 11 with reference to Example 1, purify on a Q Sepharose chromatography column (30×1.6 cm), and perform ESI- The molecular weight measured by MS is consistent with the theoretical value; then LiOH is treated to remove trifluoroacetyl, NST is catalyzed for N-sulfation modification to obtain heparin nonasaccharide 12, and its purity is >99% as measured by PAMN-HPLC, and its The molecular weight is consistent with the theoretical value.
[0092] The obtained heparin nonasaccharide 12 was modified by 2OST enzymatic method with reference to Example 1, and the reaction solution was purified by Q Sepharose chromatography column (30×1.6cm) to obtain product 13, whose component was 2303.2Da as measured by ESI-MS, indica...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Ic50 | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com