Selective hydrogenation of alkynes to alkenes in the presence of a phosphorus compound and an organic sulphur compound
A compound and selective technology, applied in metal/metal oxide/metal hydroxide catalysts, organic chemistry, compounds of Group 5/15 elements of the periodic table, etc., can solve problems such as difficult separation of compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0178] The present invention is further illustrated by the following experiments.
[0179] List of additives used in the examples:
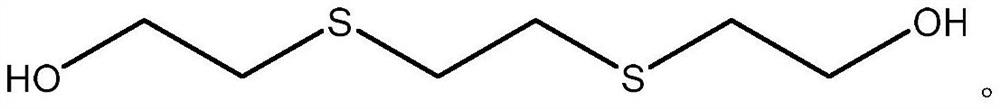
[0180] AS1 2,2'-(Ethane-1,2-diylbis(sulfanediyl))-bis(ethane-1-ol) AP1 Triphenylphosphine AP2 1,2-Bis(diphenylphosphine)ethane AP3 1,3-Bis(diphenylphosphino)propane ASP Triphenylphosphine sulfur (Ph 3 P=S)
[0181] Selective hydrogenation series 1:
[0182] Hydrogenation of methylbut-3-yn-2-ol to methylbut-3-en-2-ol
[0183] The hydrogenation catalyst (80 mg, palladium-lead on calcium carbonate containing 5% palladium by weight) was placed in a 500 ml pressure reactor. The corresponding additives in the amounts given in Table 1 and a total of 270 g of 2-methylbut-3-yn-2-ol were added to the reactor. The vessel was sealed and purged 3 times with nitrogen (pressurized to 6 bara and released). The reactor was heated to 70°C and purged 3 times with hydrogen (pressurized to 4 bara and rele...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap