Substituted tricyclics and method of use
A substituent, monocyclic technology applied in the field of substituted tricyclics and uses, which can solve problems such as limited treatment options
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
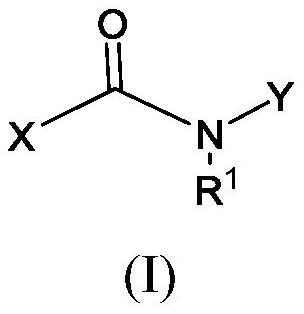
[1432] 1. a compound with formula (I) or a pharmaceutically acceptable salt thereof,
[1433]
[1434] in
[1435] R 1 is H or C 1 -C 3 alkyl;
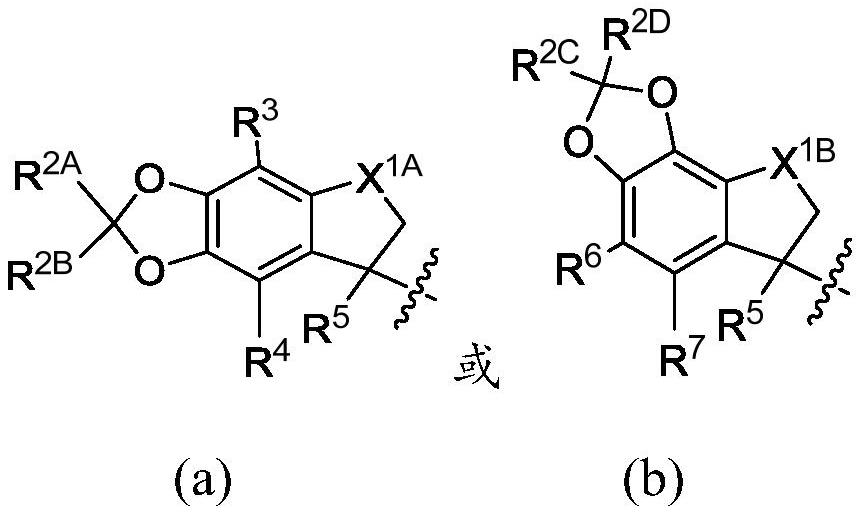
[1436] X is formula (a) or formula (b)
[1437]
[1438] in
[1439] R 2A , R 2B , R 2C , and R 2D each independently hydrogen or halogen;
[1440] R 3 , R 4 , R 6 , and R 7 independently hydrogen, C 1 -C 3 alkyl, or halogen;
[1441] R 5 independently at each occurrence hydrogen, C 1 -C 3 Alkyl, C 2 -C 4 Alkenyl, or C 1 -C 3 haloalkyl;
[1442] X 1A is O or CH 2 ;
[1443] X 1B is O or CH 2 ;
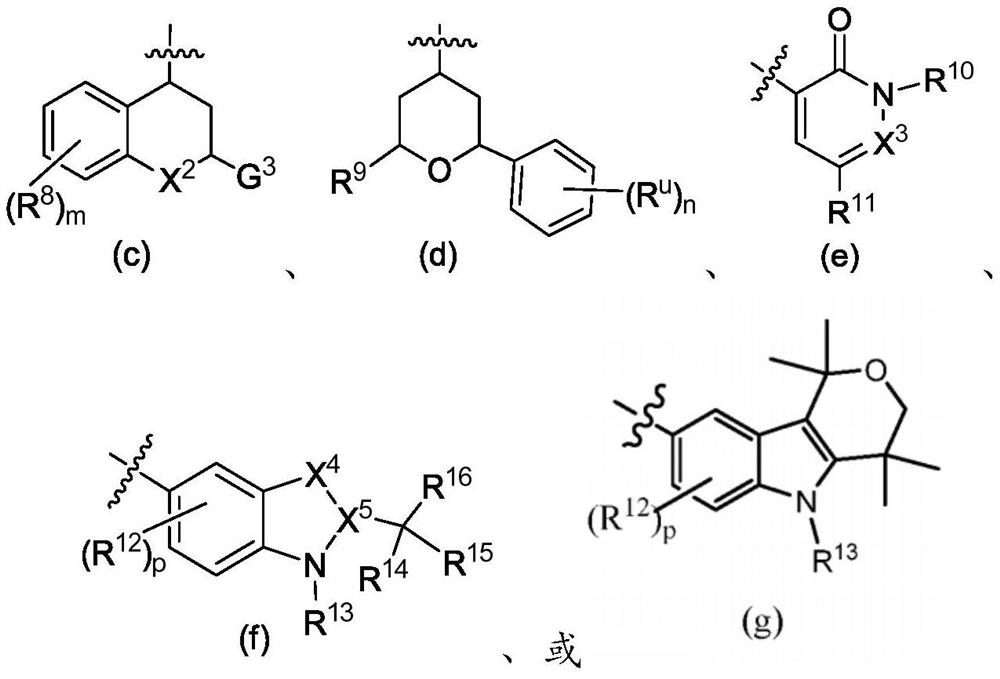
[1444] Y is -G 1 , or Y is formula (c), (d), (e), (f), or (g);
[1445]
[1446] in
[1447] G 1 is phenyl or monocyclic heteroaryl, each of which is optionally followed by 1, 2 or 3 independently selected R p group substitution; where each R p independently is C 1 -C 6 Alkyl, halogen, C 1 -C 6 haloalkyl, G 2 , C 1 -C 3 Alkoxy, C 1 -C 3 Haloalkoxy, -C(O)-G A , C(O)NR A R B , or -NR A R ...
example
[2074]All reagents were commercial grade and used as received without further purification unless otherwise indicated. Commercially available anhydrous solvents were used for the reactions carried out under an inert atmosphere. Reagent grade solvents were used in all other cases unless otherwise specified. 1 The chemical shifts (δ) of the H NMR spectra are expressed relative to tetramethylsilane as an internal standard (δ 0.00) or the appropriate residual solvent peak (i.e. CHCl). 3 (δ7.27)) in parts per million (ppm). Multiplicities are given as singlet (S), doublet (d), triplet (t), quartet (q), quintet (quin), multiplet (m) and broad (br). For microwave heating The initiator is carried out.
[2075] Enantiomeric purity was determined on an Agilent HP1100 system with UV detection. Column used: ChiralpakIA (4.6×250 mm, 5 μm). Solvents used: isopropanol and tert-butyl methyl ether.
[2076] Preparative LC / MS method TFA6
[2077] Samples were analyzed by reversed-phase...
example 1
[2085] tert-Butyl 3-{6-[(2,2-difluoro-7-methyl-6,7-dihydro-2H-furo[2,3-f][1,3]benzodioxy Heterocyclopentene-7-carbonyl)amino]-3-methylpyridin-2-yl}benzoate
PUM
| Property | Measurement | Unit |
|---|---|---|
| Linear gradient | aaaaa | aaaaa |
| Linear gradient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com