Clinical submitted data comparison method and device, computer equipment and storage medium
A data comparison and computer program technology, applied in the field of clinical trials, can solve the problems of large labor costs and time costs, achieve the effects of solving labor costs and time costs, improving user experience, reducing operating costs and difficulty of operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0023] The following will clearly and completely describe the technical solutions in the embodiments of this specification in conjunction with the drawings in the embodiments of the specification. Obviously, the described embodiments are only part of the implementations of this specification, not all of them. Based on the implementations in this specification, all other implementations obtained by those of ordinary skill in the art without making creative efforts fall within the scope of this specification.
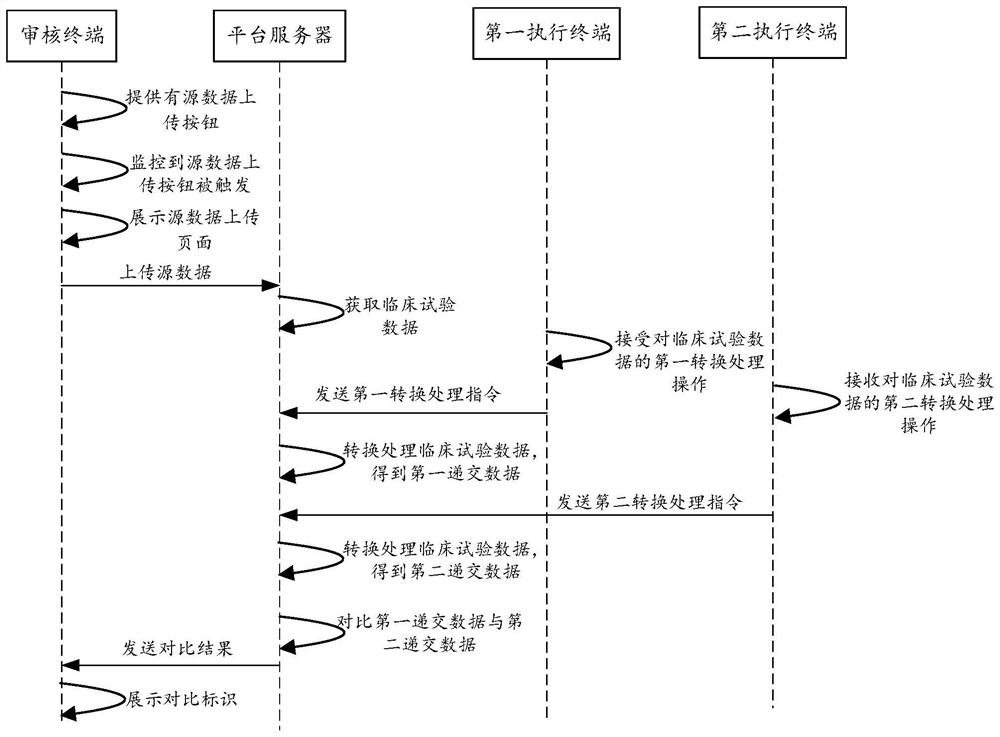
[0024] Some terms involved in this specification are explained below, and the source data (or data source) may be clinical trial data generated during the clinical trial. Source data can be case form data from EDC (Electronic Data Capture System, electronic data capture system) (such as demographic attribute data, adverse event form, combined / previous medication form, PK sample collection form, past medical history data, blood donation history, allergy history, medication...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com