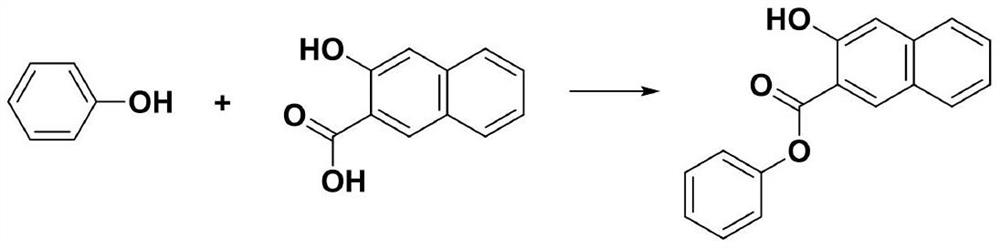
Preparation method of 3-hydroxy-2-phenyl naphthoate
A technology of naphthoic acid and hydroxyl group is applied in the field of preparation of 3-hydroxy-2-naphthoic acid phenyl ester, which can solve the problems of low yield and low product purity, and achieve the effect of important economic and social significance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] In the reaction vessel, install a stirrer, a thermometer, and a reflux condenser, add 4.5mol of dimethyl sulfoxide, 1.0mol of 2-hydroxy-3-naphthoic acid, 1.25mol of phenol, and 1-(3-dimethylaminopropyl) -3-Ethylcarbodiimide hydrochloride (EDCI) 0.25mol, heat up to reflux for 4 hours, distill off the dimethyl sulfoxide solvent, then cool the solution to 70-80°C, cool and filter, filter cake with heat After washing with sodium bicarbonate solution, washing with water until neutral, and drying, 238.9 g of phenyl 3-hydroxy-2-naphthoate were obtained as a solid product (90.4% yield, 98.6% purity).
Embodiment 2
[0018] In the reaction vessel, install a stirrer, a thermometer, and a reflux condenser, add 4.5mol of dimethyl sulfoxide, 1.0mol of 2-hydroxy-3-naphthoic acid, 1.25mol of phenol, and 1-(3-dimethylaminopropyl) -3-Ethylcarbodiimide hydrochloride (EDCI) 0.25mol, heat up to reflux for 5 hours, distill off the dimethyl sulfoxide solvent, then cool the solution to 70-80°C, cool and filter, and heat the filter cake After washing with sodium bicarbonate solution, washing with water until neutral, and drying, 243.9 g of phenyl 3-hydroxy-2-naphthoate were obtained as a solid product (92.3% yield, 98.3% purity).
Embodiment 3
[0020] In the reaction vessel, install a stirrer, a thermometer, and a reflux condenser, add 4.0mol of dimethyl sulfoxide, 1.0mol of 2-hydroxy-3-naphthoic acid, 1.20mol of phenol, and 1-(3-dimethylaminopropyl) -3-Ethylcarbodiimide hydrochloride (EDCI) 0.20mol, heat up to reflux for 4 hours, distill off the dimethyl sulfoxide solvent, then cool the solution to 70-80°C, cool and filter, filter cake with hot After washing with sodium bicarbonate solution, washing with water until neutral, and drying, 240.8 g of phenyl 3-hydroxy-2-naphthoate were obtained as a solid product (91.1% yield, 98.7% purity).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com