Method for catalytically synthesizing N-acylamino acid surfactant based on zirconium catalyst
An acylamino acid and surfactant technology, applied in the field of catalytic synthesis of fine chemicals, can solve the problems of low biodegradability, unsustainable resources, strong aquatic toxicity, etc., achieve high yield, simple steps, and avoid the synthesis process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
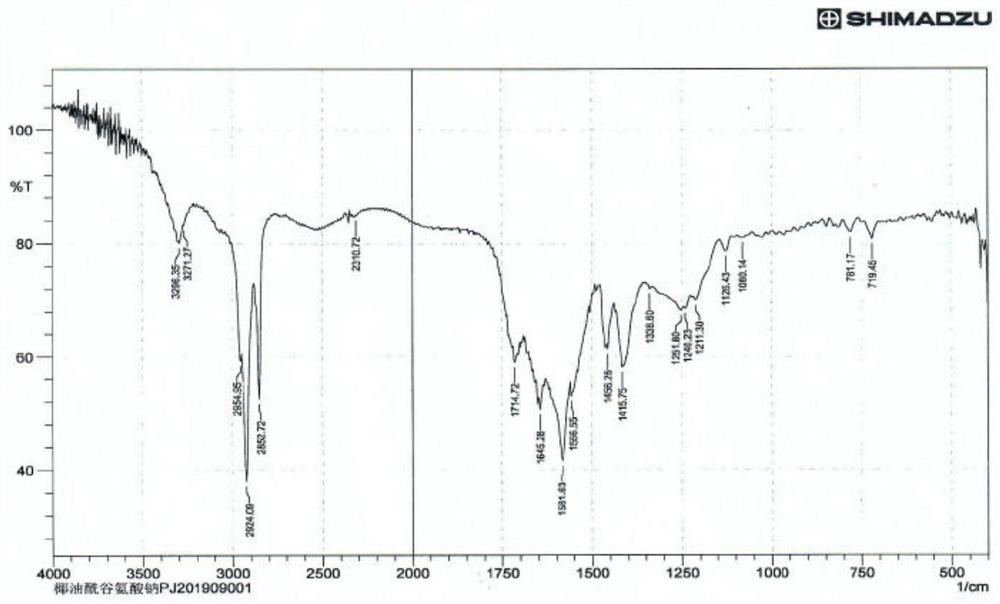
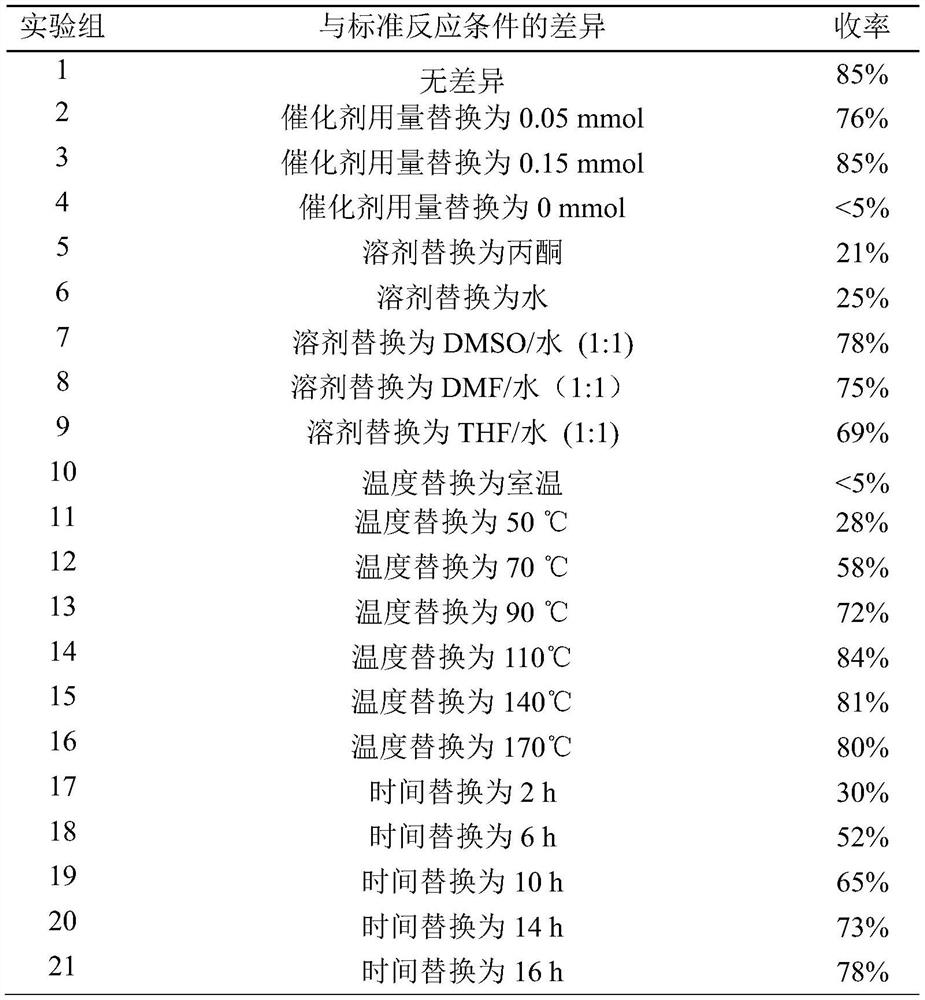
Image
Examples
Embodiment 1~41
[0035] The following examples 1 to 41 are based on lauric acid, myristic acid, palmitoleic acid, stearic acid or coconut oil, and glutamic acid or glutamate, glycine or glycinate, alanine or alanine , sarcosine or sarcosinate, methyl taurine or methyl taurine between the carboxyl-amino condensation reaction as an example to illustrate:
[0036] The N-acyl amino acid surfactants synthesized in the following examples are: glutamic acid compounds II-A~II-D, glycine compounds III-A~III-C, alanine compounds IV-A~II-B , sarcosine compounds Ⅴ-A~V-D, methyl taurine compounds VI-A~VI-C:
[0037]
[0038] where the R 1 , R 2 , R 3 , R 4 , R 5 It is undecyl, tridecyl, pentadecyl, 7-pentadecenyl, heptadecyl or 9-heptadecenyl.
Embodiment 1
[0040] Add catalyst bis(perfluorohexylsulfonate) zirconocene (0.0888g, 0.1mmol), lauric acid (2.000g, 10mmol), sodium glutamate (1.6911g, 10mmol) and acetone / Water (1:1) mixed solvent (30mL), reacted at 100°C for 24h, stopped the reaction, removed the solvent, added ethanol / petroleum ether mixed solvent to the solid compound, and crystallized for 24h to obtain sodium lauroyl glutamate with a yield of 85% .
Embodiment 2
[0042] A round bottom flask (100 mL) was charged with catalyst bis(perfluorohexylsulfonate) zirconocene (0.0888 g, 0.1 mmol), lauric acid (2.000 g, 10 mmol), disodium glutamate (1.9109 g, 10 mmol) and acetone / water mixed solvent (30mL), reacted at 100°C for 24h, stopped the reaction, removed the solvent and catalyst to obtain disodium lauroyl glutamate with a yield of 77%.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap