Application of flavonoid USP22 inhibitor in preparation of anti-tumor immune drugs
A technology of flavonoids and anti-tumor immunity, applied in the field of anti-tumor immune drugs, can solve the problems of high-throughput screening of USP22 small-molecule inhibitors, difficulty in obtaining USP22 crystal structure, and lack of co-crystal structure data reports, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Molecular Interaction Analysis of Baohuoside I, Moranginin and Chrysin with USP22 Protein
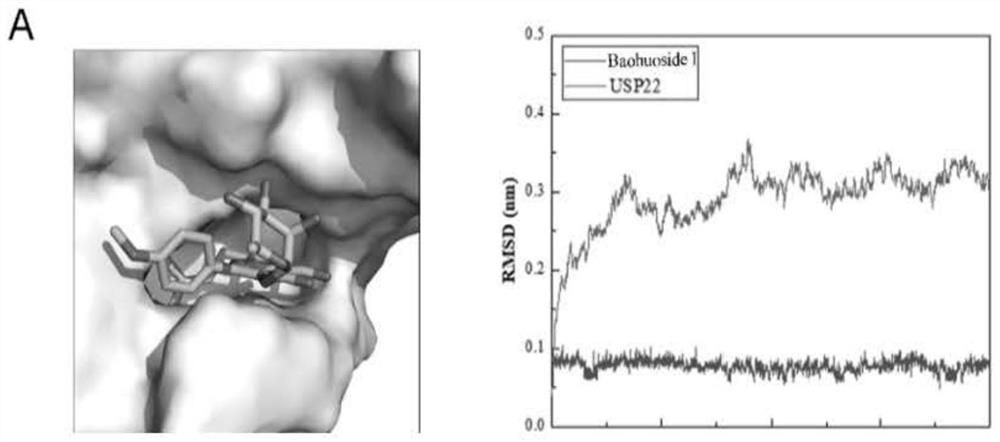
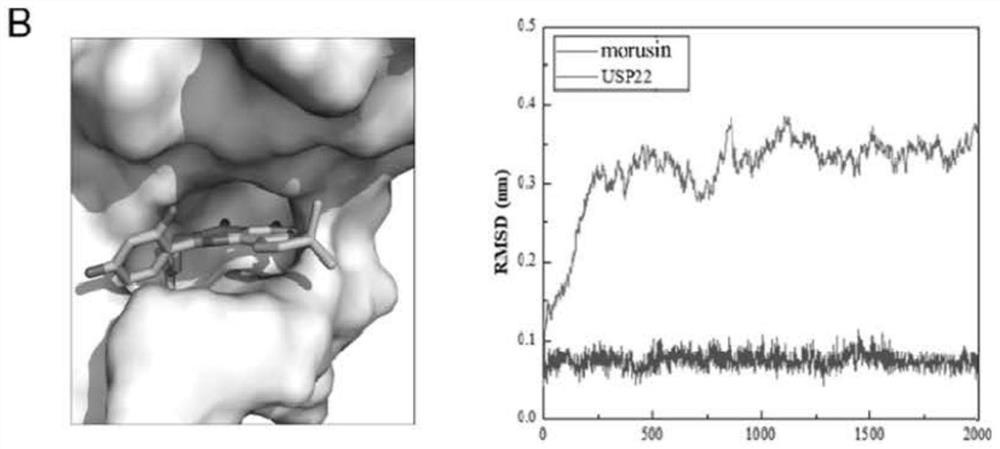
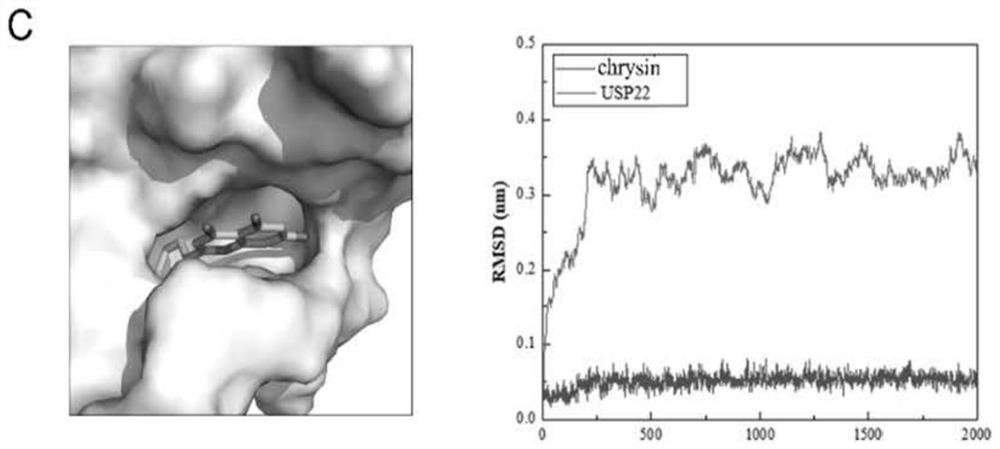
[0035] We used the homology modeling method to construct the structure of human USP22 protein. Based on this structure model, we used the computer-aided drug design method to screen the natural compound library and found that flavonoids have the potential to inhibit the functional activity of USP22. Molecular docking results showed that baohuoside I, sangsin, and chrysin matched with the pocket of the catalytic domain of USP22 protein receptor. Such as Figure 1-A The figure on the left shows that the stick-shaped structure is the 3D structure of baojoside I, which is stably bound in the pocket of the catalytic domain of the USP22 protein receptor. In order to further understand whether baojoside I binds to USP22 protein stably, we used molecular dynamics simulation to analyze the RMSD value (root mean square deviations, root mean square deviation), such as Figure 1-A As shown ...
Embodiment 2
[0038] Baohuoside I, Moranginin and Chrysin Inhibit the Functional Activity of USP22
[0039]In the hSAGA complex, DUBm binds to nucleosomes through histones H2A and H2B. Studies have shown that both histones H2A and H2B can be inhibited by USP22. The degree of priming will increase. According to the IC50 value of baohuoside I, sangxinsu and chrysin on human colon cancer HCT116 cells, the human colon cancer HCT116 cells in the logarithmic growth phase were administered with 20 μM, 8 μM and 40 μM for co-culture for 12 hours. The protein levels of monoubiquitinated H2A and H2B were detected by Western Blot method. The result is as Figure 2-A As shown, the expression of USP22 protein is almost the same as that of the control group, indicating that the compound does not affect the expression of USP22 protein. The expression of monoubiquitinated H2A (H2Aub1) and H2B (H2Bub1) proteins were both up-regulated ( Figure 2-A ).
[0040] Previous studies have shown that USP22 can d...
Embodiment 3
[0043] Baohuoside I and Sangsin specifically inhibit the functional activity of USP22
[0044] In order to verify whether the ubiquitination-promoting target of baohuoside I and morinin is USP22, we used Si-RNA interference technology to silence the expression of USP22. As a comparison, the changes of H2Bub1 in the cells of the normal administration group that were not interfered with by Si-RNA were detected at the same time, such as Figure 3-A As shown, the administration of undisturbed cells has almost no effect on the expression of USP22, while the expression of H2Bub1 in the administration group is significantly increased compared with that in the no-drug group. Such as Figure 3-B As shown, when HCT116 cells were interfered with Si-RNA, the H2Bub1 of the interfered cells was significantly increased compared with the control group, while the Baohuoside I and Sangsin administration groups basically did not further promote the H2B activity compared with the Si-RNA group. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com