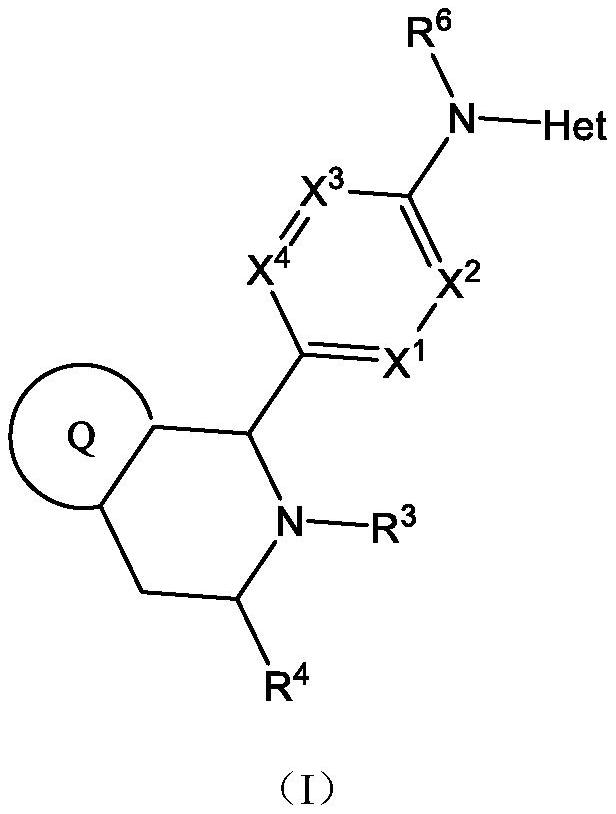
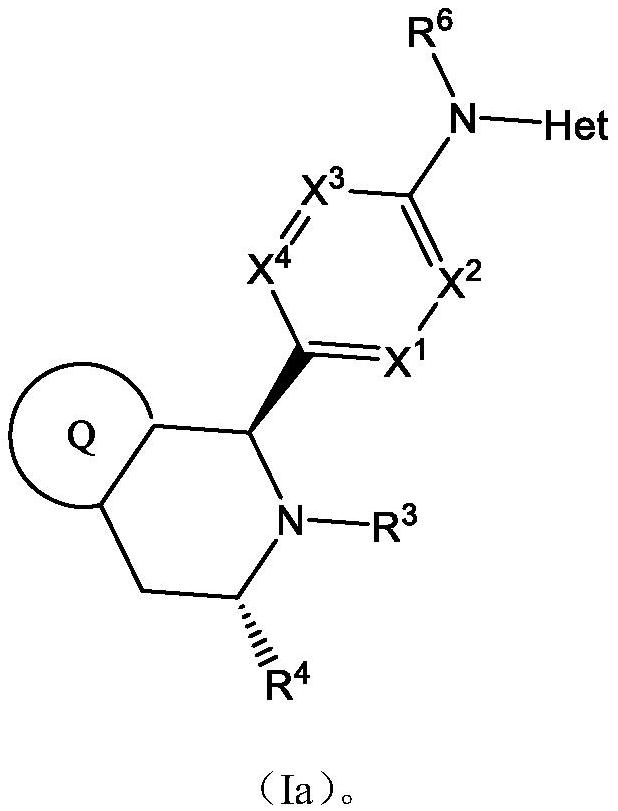
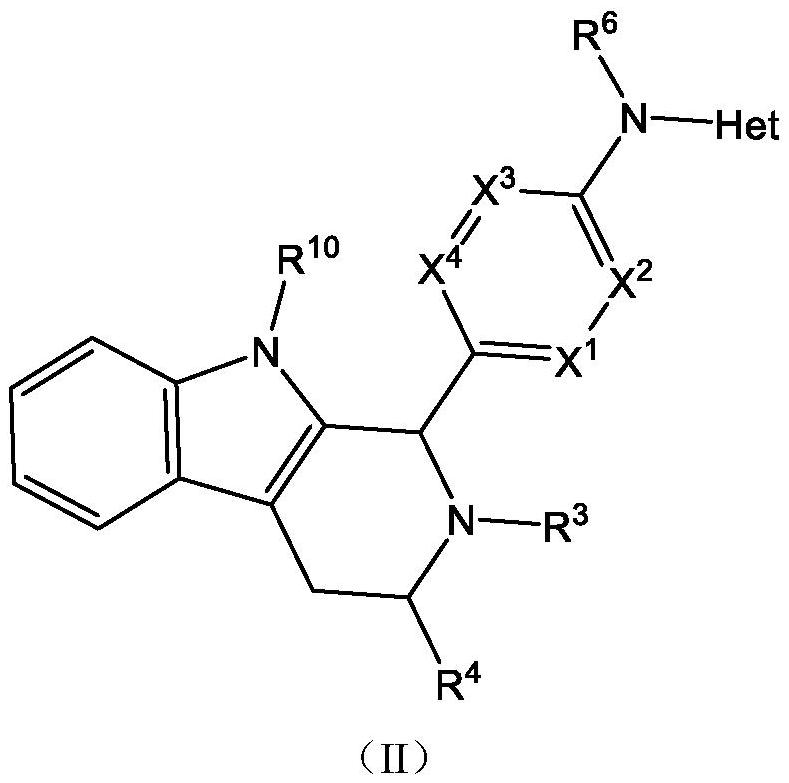
Estrogen receptor modulator compounds and uses thereof
A compound and selected technology, applied in the fields of organic chemistry, drug combination, organic chemistry methods, etc., can solve the problems of limiting the efficacy of the highest dose and failing to fully realize the degradation of ER.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0131] Example 1: 3-(((1R,3R)-1-(2,6-difluoro-4-((1-((((1R,2R)-2-fluorocyclopropyl)methyl)nitrogen Heterobutan-3-yl)amino)phenyl)-3-methyl-1,3,4,9-tetrahydro-2H-pyrido[3,4-b]indol-2-yl)- 2,2-Difluoropropan-1-ol
[0132]
[0133] Step 1: Synthesis of 5-bromo-2-(diethoxymethyl)-1,3-difluorobenzene
[0134]
[0135] Add 4-bromo-2,6-difluorobenzaldehyde (2.19g, 10.0mmol) into 50mL ethanol, add triethyl orthoformate (1.48g, 10.0mmol), add 1 drop of concentrated sulfuric acid and heat to 60 After stirring at ℃, the reaction was completed after 4 hours, and the title compound was obtained after the reaction solution was spin-dried.
[0136] Step 2: Synthesis of tert-butyl 3-(((4-(diethoxymethyl)-3,5-difluorophenyl)amino)azetidine-1-carboxylate
[0137]
[0138] 5-Bromo-2-(diethoxymethyl)-1,3-difluorobenzene (2.94g, 10.0mmol), 1-tert-butoxycarbonyl-3-aminocyclobutylamine (2.06g, 12.0 mmol), tris(dibenzylideneacetone)dipalladium (458mg, 0.5mmol) 4,5-bisdiphenylphosphine-9,...
Embodiment 2
[0156] Example 2: 3-(((1R,3R)-1-(2,6-difluoro-4-((1-((((1S,2S)-2-fluorocyclopropyl)methyl)nitrogen Heterobutan-3-yl)amino)phenyl)-3-methyl-1,3,4,9-tetrahydro-2H-pyrido[3,4-b]indol-2-yl)- 2,2-Difluoropropan-1-ol
[0157]
[0158] The preparation method is similar to Example 1, except that (1R, 2R)-2-fluorocyclopropane-1-carboxylic acid in step 6 in Example 1 is replaced by (1S, 2S)-2-fluorocyclopropane- 1-Carboxylic acid, the last two-step reaction of Example 1 was carried out in the same way to obtain the title compound.
[0159] DMSO-d6δ H 10.50(s,1H),7.38-7.36(m,1H),7.19-7.17(m,1H),7.00-6.91(m,2H),6.70-6.68(m,1H),6.11-6.08(m,2H ),5.25-5.20(m,1H),5.05(s,1H),4.82-4.78(m,0.5H),4.65-4.62(m,0.5H),3.95-3.92(m,1H),3.69-3.60 (m,3H),3.47-3.37(m,2H),3.14-3.03(m,1H),2.85-2.80(m,3H),2.64-2.50(m,3H),2.47-2.42(m,1H) ,1.07-1.05(m,3H),0.91-0.85(m,1H),0.79-0.71(m,1H),0.59-0.49(m,1H).
[0160] LC / MS (m / z, MH + ):535.2
Embodiment 3
[0161] Example 3: 3-(((1R,3R)-1-(2,6-difluoro-4-((1-((((1S,2R)-2-fluorocyclopropyl)methyl)nitrogen Heterobutan-3-yl)amino)phenyl)-3-methyl-1,3,4,9-tetrahydro-2H-pyrido[3,4-b]indol-2-yl)- 2,2-Difluoropropan-1-ol
[0162]
[0163] The preparation method is similar to Example 1, except that (1R, 2R)-2-fluorocyclopropane-1-carboxylic acid in step 6 in Example 1 is replaced by (1S, 2R)-2-fluorocyclopropane- 1-Carboxylic acid, the last two-step reaction of Example 1 was carried out in the same way to obtain the title compound.
[0164] DMSO-d6δ H 10.51(s,1H),7.38-7.35(m,1H),7.19-7.17(m,1H),7.00-6.91(m,2H),6.70-6.68(m,1H),6.11-6.08(m,2H ),5.25-5.20(m,1H),5.05(s,1H),4.82-4.78(m,0.5H),4.65-4.62(m,0.5H),3.95-3.92(m,1H),3.69-3.60 (m,3H),3.47-3.37(m,2H),3.14-3.03(m,1H),2.85-2.80(m,3H),2.64-2.49(m,3H),2.47-2.42(m,1H) ,1.07-1.05(m,3H),0.91-0.85(m,1H),0.79-0.71(m,1H),0.59-0.49(m,1H).
[0165] LC / MS (m / z, MH+):535.2
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com