Triple inactivated vaccine for preventing and treating duck circovirus disease, novel duck reovirus disease and duck adenovirus type 3 and preparation method of triple inactivated vaccine
A duck circovirus, reovirus technology, applied in chemical instruments and methods, biochemical equipment and methods, vaccines, etc., can solve problems such as ineffectiveness, achieve low cost, high vaccine titer content, and preparation methods simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
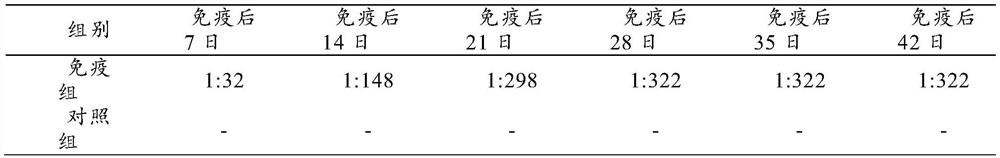
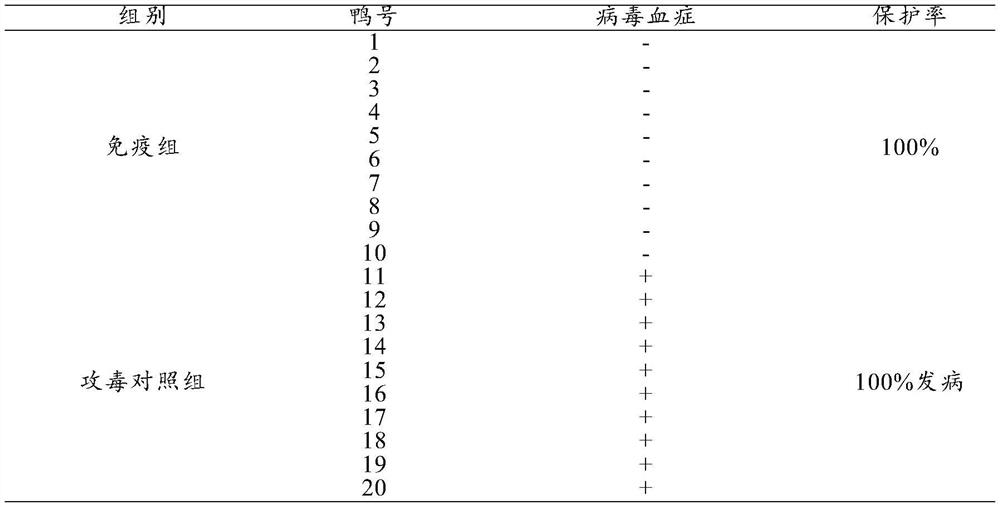
[0030] Preparation of Example 1 Triple Inactivated Vaccine for Duck Circovirus Disease, New Duck Reovirus Disease and Type 3 Duck Adenovirus Disease
[0031] 1. Poison species
[0032] Novel duck reovirus S strain (microorganism preservation number: CGMCC No.20000, its classification name is: new duck reovirus, preservation time is: July 6, 2020, preservation unit is: China Microbial Strains The General Microbiology Center of the Preservation Management Committee, the preservation address is: No. 3, No. 1 Courtyard, Beichen West Road, Chaoyang District, Beijing, Institute of Microbiology, Chinese Academy of Sciences) is separated, identified, kept and supplied by Harbin Pharmaceutical Group Biological Vaccine Co., Ltd.
[0033] 2. Preparation and testing of inactivated vaccines
[0034] 2.1 Preparation of duck circobaculovirus
[0035] 2.1.1 Design and synthesis of duck circovirus CAP gene
[0036] After the duck circovirus cap gene was codon-optimized, EcoRI and Hind III r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com