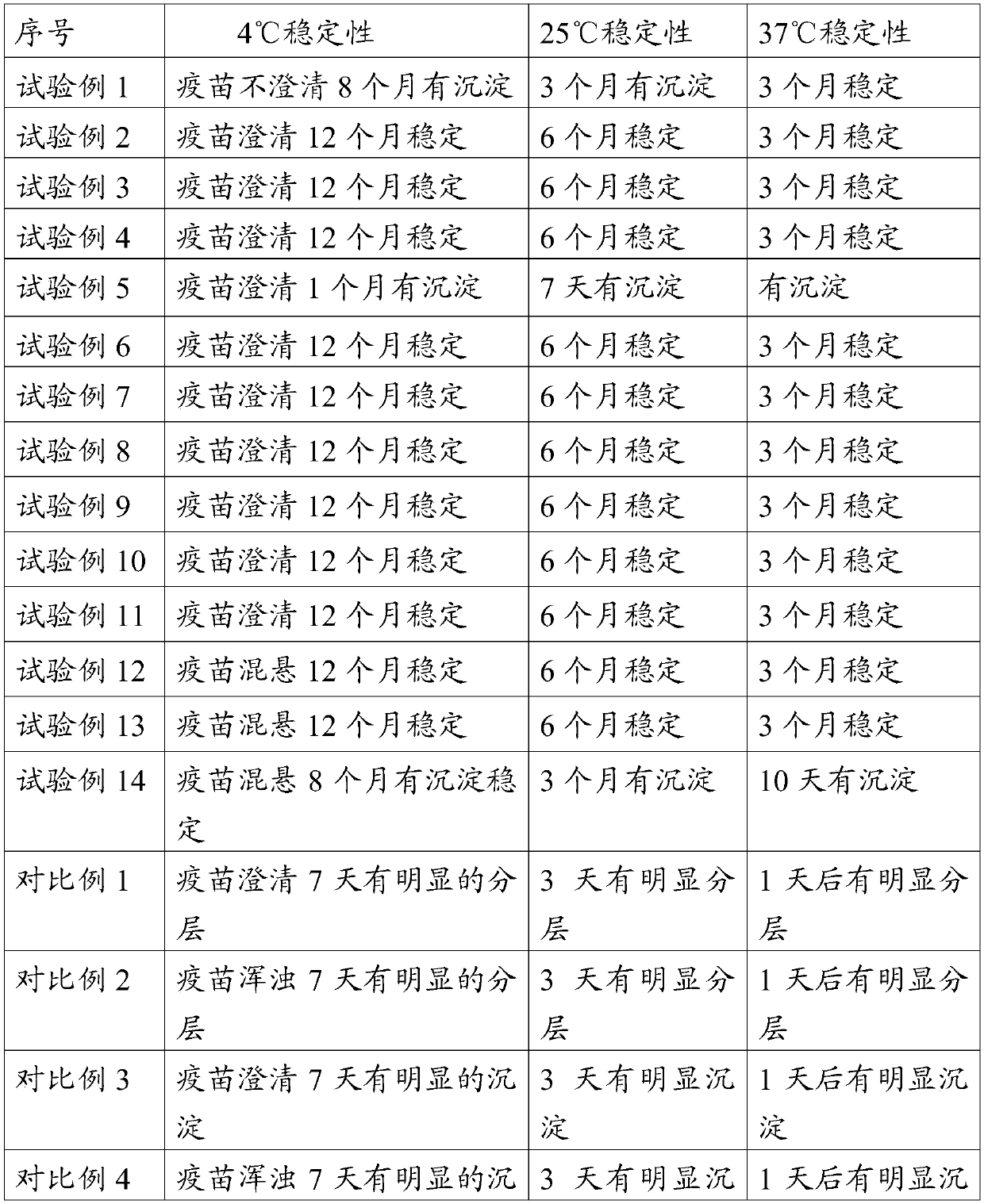
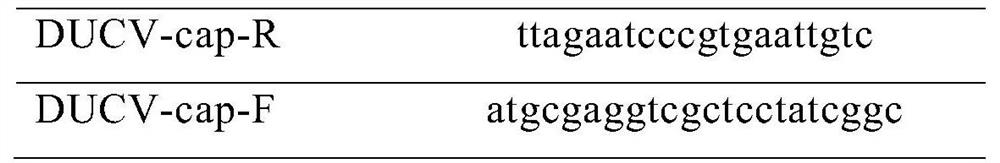
Patents
Literature
38 results about "Leb antigen" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
C. perfringens alpha toxoid vaccine
Owner:SCHERING PLOUGH LTD +2
Water-in-oil-in-water adjuvant vaccine and preparation method thereof
ActiveCN103223164APromote absorptionGenerate fastAntiinfectivesAntibody medical ingredientsEngineeringImmunity response
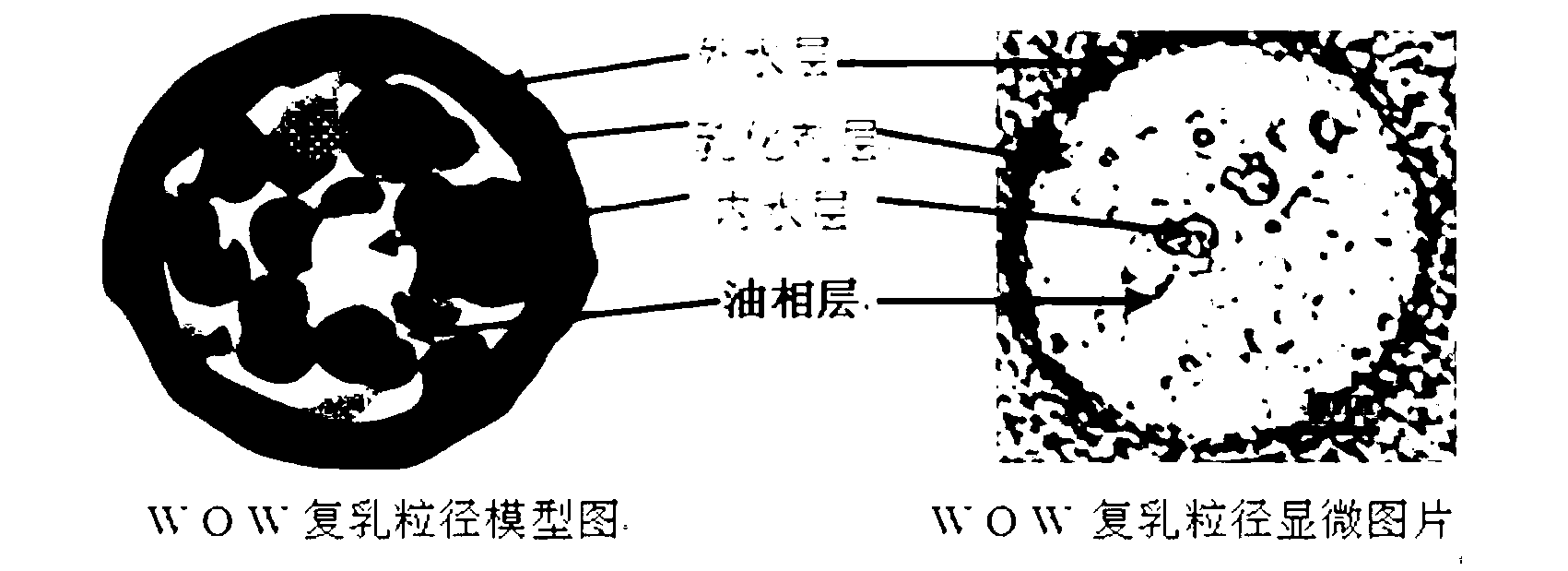
The invention discloses a water-in-oil-in-water adjuvant vaccine and a preparation method thereof. The adjuvant vaccine is composed of an internal water phase, a middle oil phase used for cladding the internal water phase, and an external water phase for cladding the middle oil phase. The external water phase comprises: a composite surfactant, a hydrophilic surfactant and mormal saline. The middle oil phase consists of: a lipophilic surfactant, a stabilizer and mineral oil. The internal water phase comprises: an immunopotentiator solution, the hydrophilic surfactant, and an inactivated antigen solution. The W / O / W (water-in-oil-in-water) type adjuvant vaccine provided in the invention can induce an immunoreaction earlier than traditional W / O (water-in-oil) type adjuvant vaccines, and can make experimental animals obtain earlier protective antibodies. The vaccine has good absorption, and causes a small inflammatory response at an inoculation site. The adjuvant vaccine disclosed in the invention has better stability than existing W / O / W type vaccines, and the antibody generation speed is fast.
Owner:SOUTH CHINA AGRI UNIV +1
Inactivated vaccine of cow chlamydia, its preparation and inspection method
ActiveCN1698892AFight infectionInfection fromChlamydiaceae ingredientsAntiinfectivesMicrocosmic saltOil adjuvant
The invention relates to an inactivated vaccine of cow chlamydia, its preparation and the related inspection method during the vaccine preparation. The preparing process comprises diluting the Chlamydia psittaci SX 5 or NX with microcosmic salt buffering liquid or physiological saline, vaccinating to healthy chick embryo hatched at 37 deg. C for 6-7 days, harvesting vitelline membrane and allantois liquid of dead chick embryo after 72 hours as antigens, triturating the antigens, diluting and charging formaldehyde for deactivation, mixing the deactivated antigens with oil adjuvant by the proportion of 1:1, stirring homogeneously, carrying out homogeneous emulsion to obtain the vaccine.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Method for determining titer of swine flu inactivated vaccine
ActiveCN102735853AReduced measurement timeReduce operating errorsBiological testingRed blood cellEngineering
The invention discloses a method for determining titer of a swine flu inactivated vaccine. The method comprises the following steps: immunizing a pig with a swine flue inactivated vaccine, collecting blood 21-28 days after immunization and separating blood serum; removing non-specific components in the serum to obtain a serum to be tested; diluting the swine flue inactivated antigen, and preparing a unit swine flue antigen diluent with a concentration of 4HA; and diluting the serum to be tested by multiple proportions, successively adding the 4HA unit swine flue antigen diluent and 1% red cells to conduct hemagglutination inhibition test, so as to completely inhibit highest dilution of the 4HA unit swine flu antigen serum at HI titer. The method has advantages of greatly shortened measurement time, little operation error, strong controllability, and small inter-batch difference, and also reduces detection errors caused by different levels of experimental animals in an animal challenge protection experiment for testing the titer.
Owner:GUANGZHOU SOUTH CHINA BIOLOGICAL MEDICINE +1
Newcastle disease and avian flu antigen-antibody complex inactivated vaccine and preparation method
ActiveCN104984337AHigh potencyImprove hydrophilicityViral antigen ingredientsAntiviralsDiseaseAntigen release
The present invention relates to a Newcastle disease and avian flu antigen-antibody complex vaccine preparation method, which comprises: preparing avian flu inactivated antigen, preparing Newcastle disease inactivated antigen and avian flu inactivated yolk antibody, respectively emulsifying the inactivated Newcastle disease antigen, the inactivated avian flu antigen and the inactivated avian flu yolk antibody semi-finished product, and mixing. According to the present invention, the Newcastle disease and avian flu antigen-antibody complex vaccine with characteristics of vaccine immunization protection capability enhancing, antigen release delaying, good safety, high purity and production cost saving, suitable for all chicken flocks and especially providing safety and effectiveness for chicks and chicken having diseases is provided.
Owner:兆丰华生物科技(南京)有限公司
Preparation and application of porcine epidemic diarrhea virus IgA antibody ELISA kit
PendingCN110967480ARealize immune effect evaluationAchieve clinical evaluationMaterial analysisElisa kitMonoclonal
The invention provides a porcine epidemic diarrhea virus IgA antibody ELISA detection kit. The kit comprises a supporting medium coated with a porcine epidemic diarrhea virus antibody-antigen compound, the porcine epidemic diarrhea virus antibody-antigen compound being a porcine epidemic diarrhea virus IgG monoclonal antibody-inactivated antigen compound, enzyme labeled anti-swine IgA second antibody, and a detection reagent, the positive control and the negative control used for detecting the reaction of the porcine IgA and the porcine epidemic diarrhea virus antibody-antigen complex antigen-antibody. The kit disclosed by the invention is high in sensitivity, and can be used for accurately detecting a plurality of target samples in milk, serum, saliva, anus swab and excrement samples.
Owner:LUOYANG PULIKE WANTAI BIOTECH
Immunologic adjuvant composition as well as preparation method and application thereof
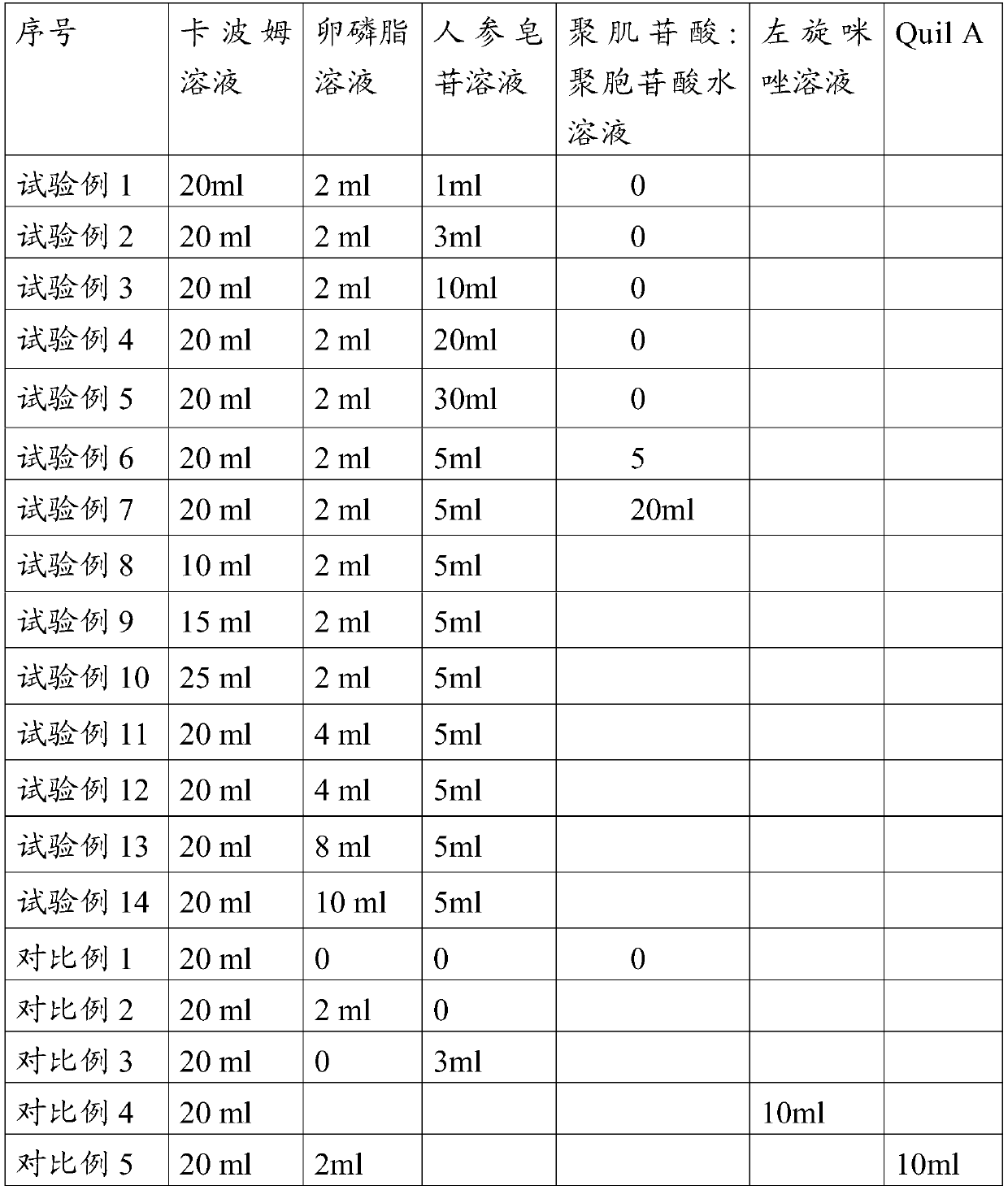
The invention provides an adjuvant composition. The adjuvant composition comprises 0.2-0.5%w / v of carbomer, 0.1-0.5%w / v of lecithin and 0.03-0.2%w / v of ginsenoside. The adjuvant composition of the invention can maintain long-term clarification and / or stability of vaccine, the inactivated antigen and subunit antigen in the composition can be effectively excited to produce high-titer antibodies forimplementing immunoprotection. The inactivated vaccine or subunit vaccine prepared by the adjuvant composition of the invention can be used as a diluent for freeze-dried live virus antigens, which hasno toxic and killing effects on live virus antigens.
Owner:LUOYANG SEIWEI BIOTECHNOLOGIES CO LTD
Streptococcus suis and haemophilus parasuis bivalent inactivated vaccine and preparation method thereof
PendingCN114181846ALittle side effectsImprove securityAntibacterial agentsBacterial antigen ingredientsAntigenAdjuvant
The invention discloses a streptococcus suis and haemophilus parasuis combined inactivated vaccine and a preparation method thereof. The streptococcus suis and haemophilus parasuis combined inactivated vaccine comprises an antigen and an adjuvant, the antigen comprises an inactivated antigen concentrated solution of a haemophilus parasuis serum type 4 YC strain, a haemophilus parasuis serum type 5 SQ strain, a streptococcus suis type 2 NT strain and a streptococcus suis type 9 CZ strain. The vaccine provided by the invention can be used for simultaneously preventing haemophilus parasuis disease caused by haemophilus parasuis, streptococcus suis disease caused by streptococcus suis and mixed infection of the haemophilus parasuis disease and the streptococcus suis disease, is small in side effect and good in safety, can achieve the effect of preventing multiple diseases by one injection, and particularly can be used for preventing and treating infection of currently popular serotype germs; comprising infection of haemophilus parasuis type 4 and type 5 and infection of streptococcus suis type 2 and type 9.
Owner:JIANGSU NANNONG HI TECH
Purification method for large-scale production of embryogenic antigens
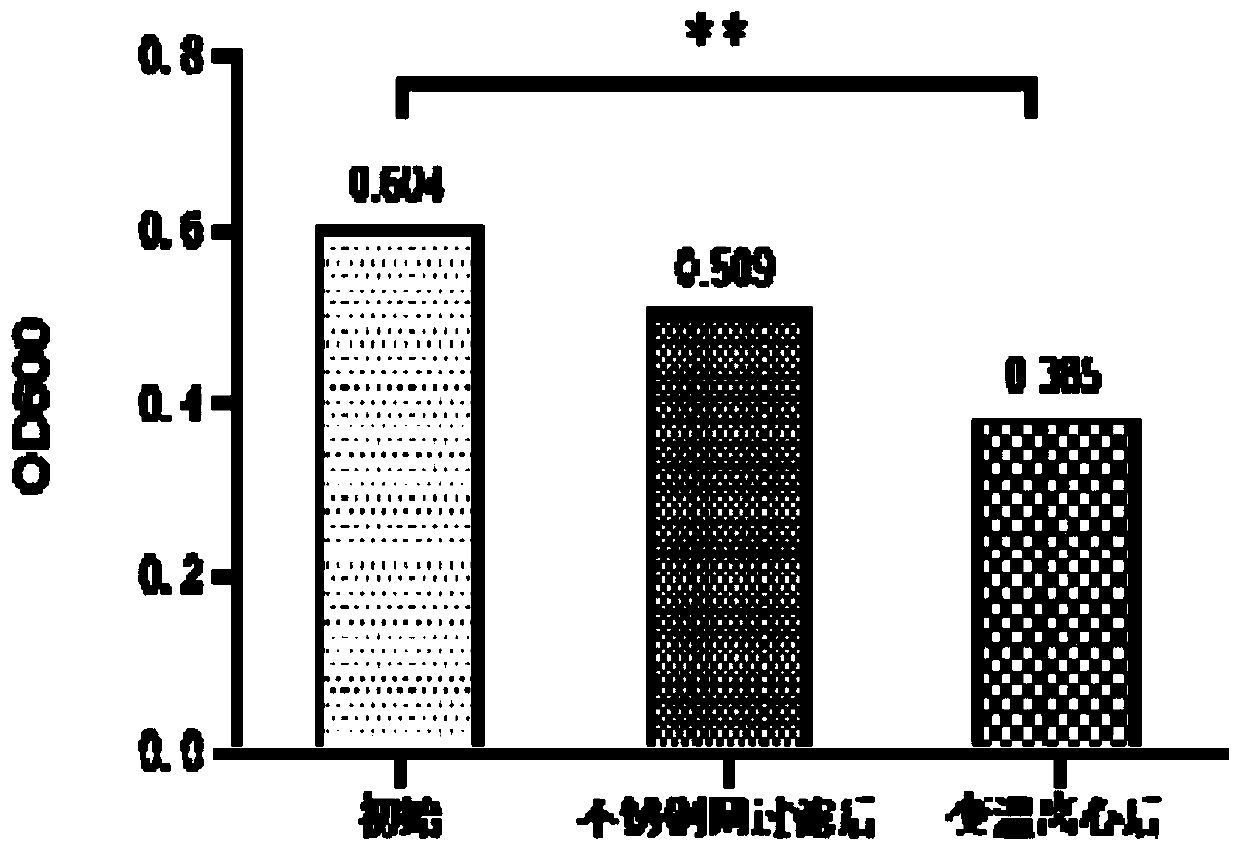
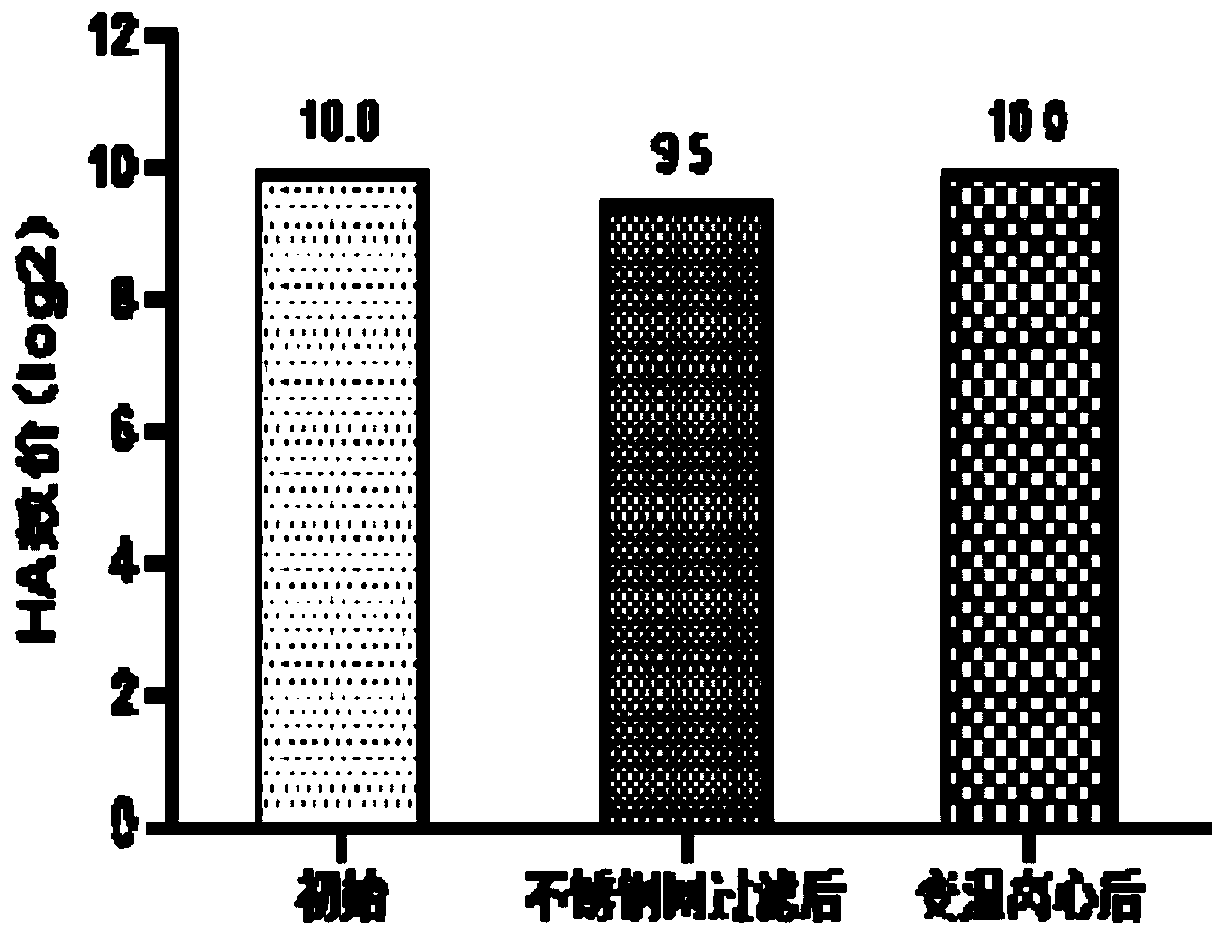
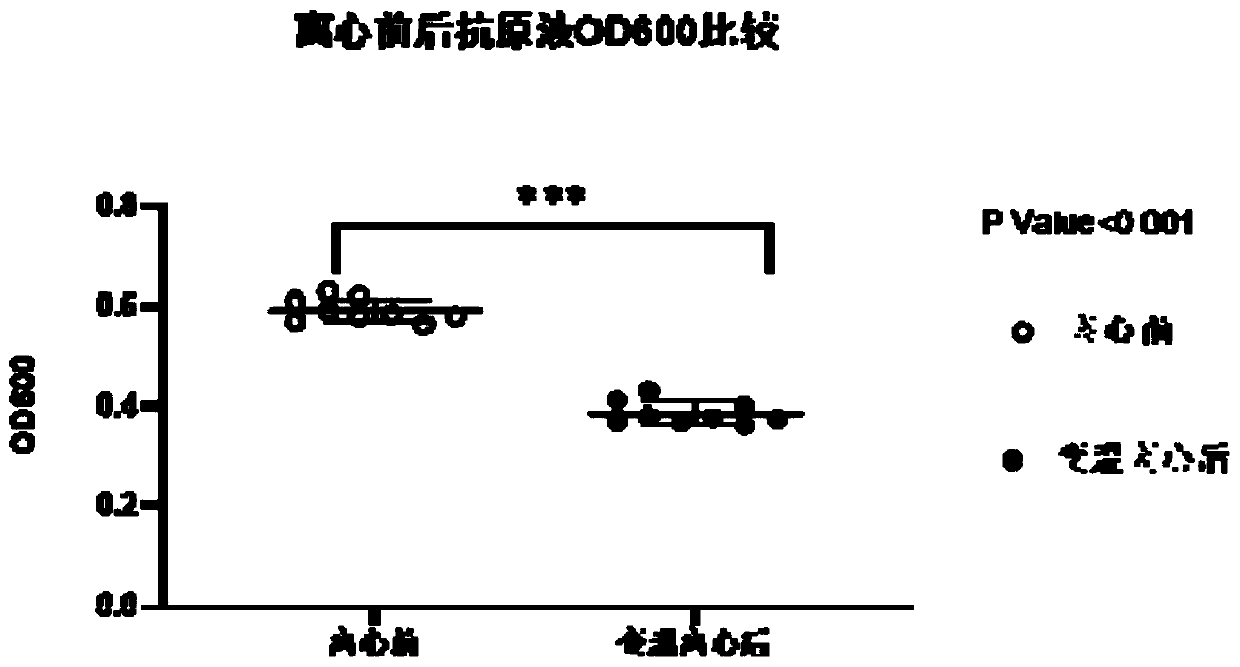
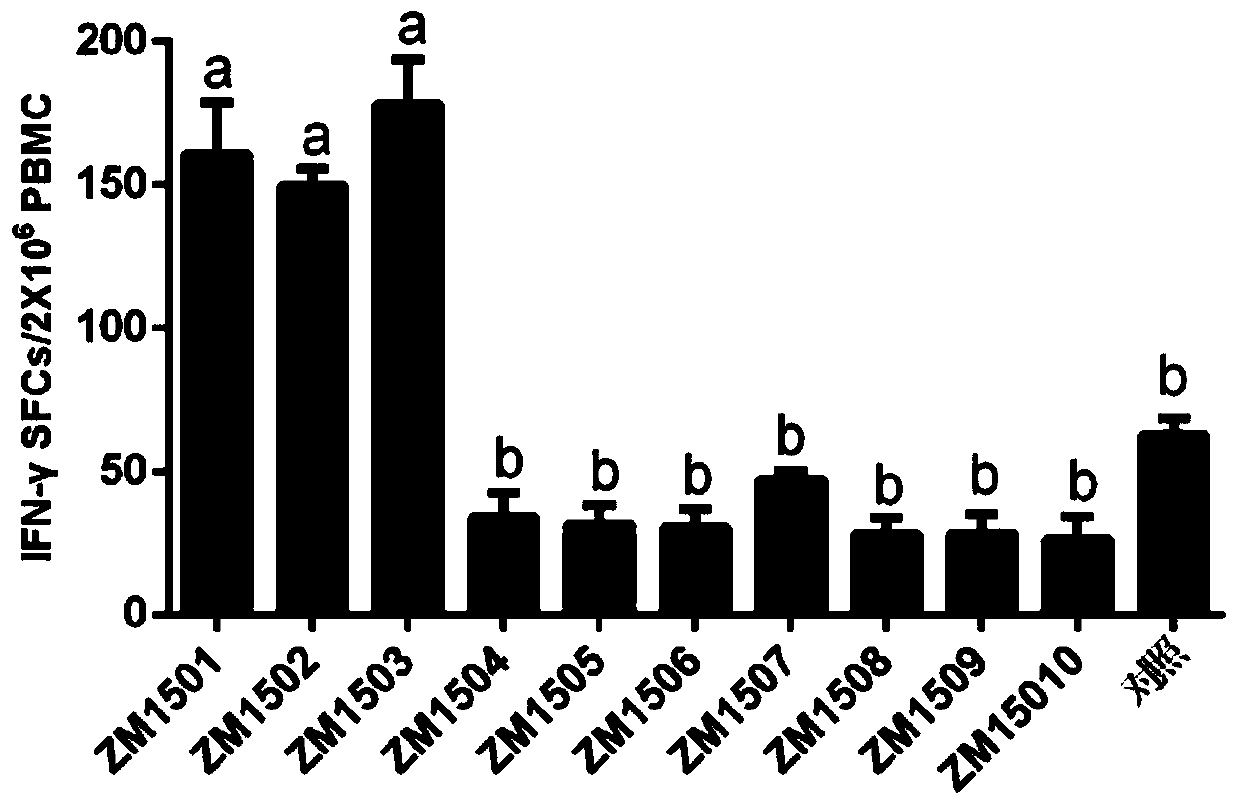
InactiveCN111303252AStructure remains intactImprove the purification effectSsRNA viruses negative-senseSsRNA viruses positive-senseLeb antigenCentrifugation
The invention provides a purification method for large-scale production of embryogenic antigens. According to the purification method, residual denatured impure proteins in inactivated antigen liquidcan be thoroughly cleared, so that the safety of a vaccine is further improved. The method comprises the following steps: carrying out cooling during inactivation of embryogenic inactivated antigens by formaldehyde; and carrying out standing, carrying out high speed centrifugation on the inactivated antigens in a centrifugal force of 13000g-16000g, so as to obtain supernate, namely the purified embryogenic antigens. According to the method, the residual impure proteins are denatured and separated through temperature varying and then are removed by virtue of a centrifugation method, and meanwhile, a complete structure of a target antigen is maintained.
Owner:YEBIO BIOENG OF QINGDAO
Duck reovirus and duck circovirus bivalent inactivated vaccine and preparation method thereof
PendingCN113384692AHigh titer contentAvoid adverse reactionsViral antigen ingredientsAntiviralsReovirus (antigen)Leb antigen
The invention discloses a duck reovirus and duck circovirus bivalent inactivated vaccine and a preparation method thereof. The bivalent inactivated vaccine comprises an effective amount of inactivated antigen and an immunologic adjuvant in immunization, wherein the inactivated antigen comprises duck reovirus antigen and duck circovirus antigen proteins. The invention further discloses a preparation method of the bivalent inactivated vaccine. The bivalent inactivated vaccine provided by the invention is good in safety, does not generate mutual interference or influence of antigen components, can achieve the protection effect of secondary immunization of each single vaccine through one-time immunization, can prevent duck reovirus and duck circovirus at the same time, and can avoid adverse reaction caused by multiple times of immunization, and the preparation method has the advantages of simplicity, convenience, multiple effects, low cost, high vaccine titer content and the like.
Owner:哈药集团生物疫苗有限公司
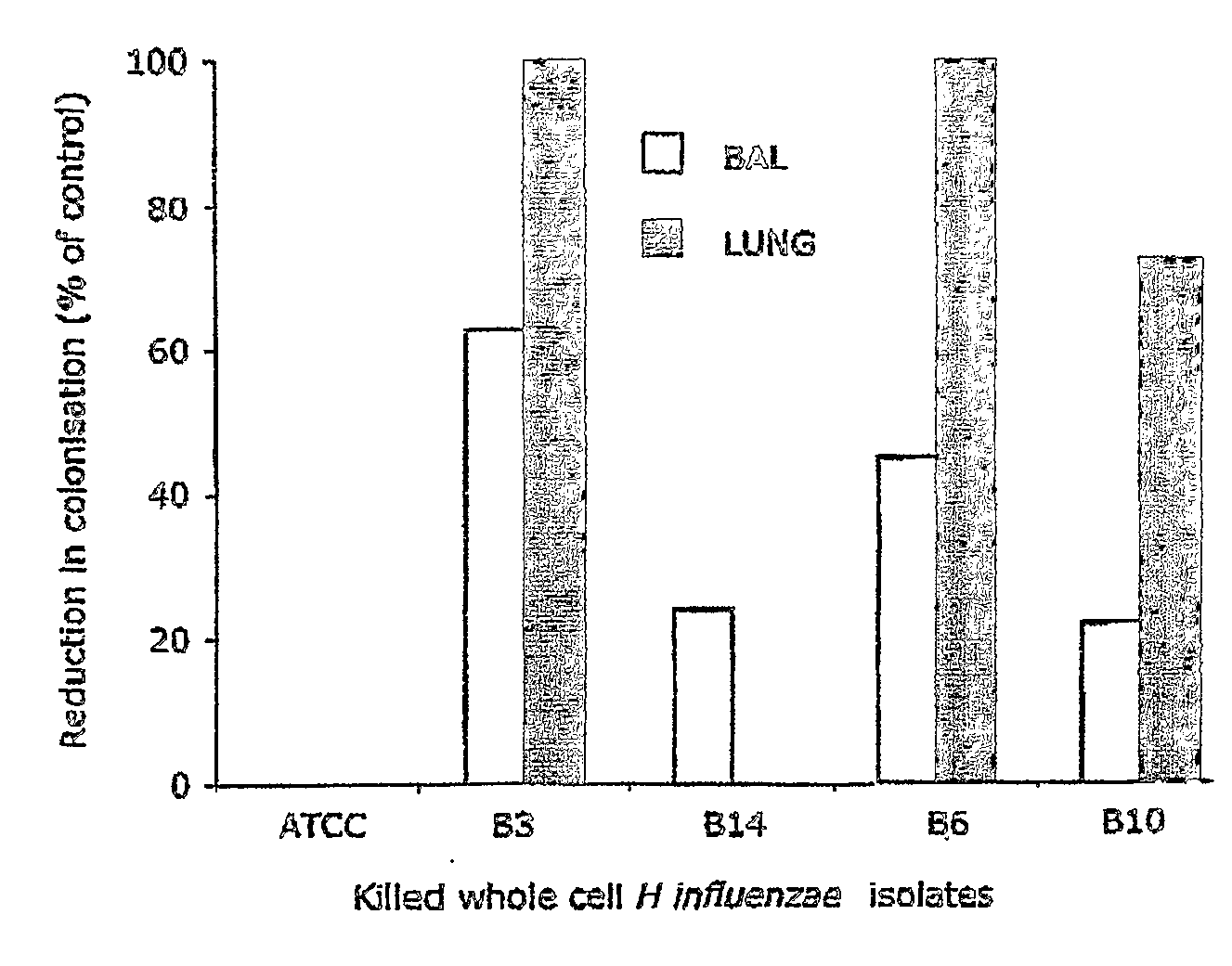
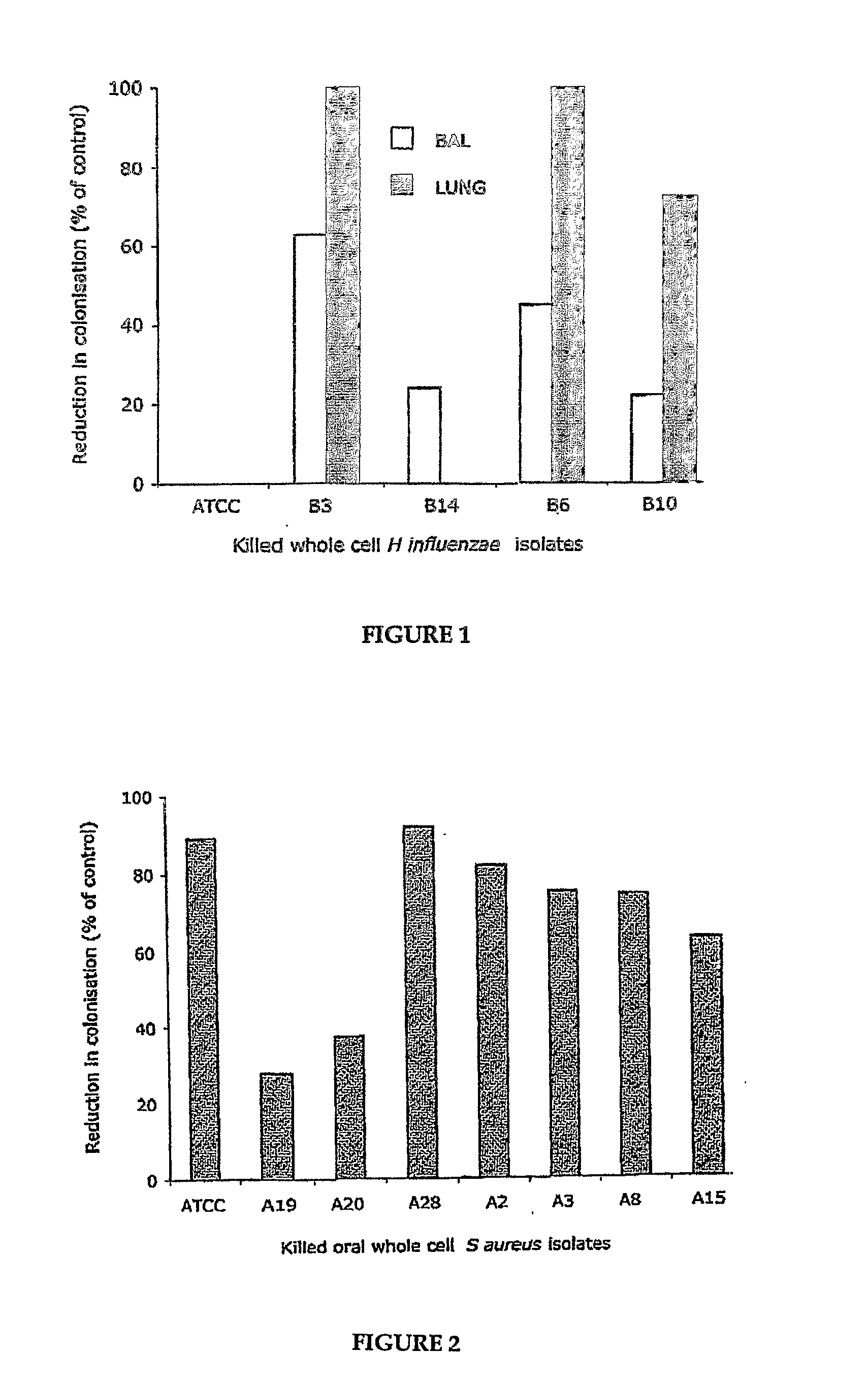
Oral Killed Vaccines and Method for Providing Same
There is described a method for selecting microbial isolates for use in oral killed vaccines against abnormal microbial colonisation of mucosal surfaces by the microbes. The method comprises evaluating capacity of a plurality of different isolates of a microbe to activate antigen responsive cells to provide activation data for each microbial isolate, and the effectiveness of the isolates in reducing infection of a mucosal surface by the microbe to provide clearance data for each microbial isolate. An isolate, the activation data and clearance data for which correlate and is optimal for generating mucosal immunity against the microbe compared to the, or each, other of the isolates, or an isolate the activation data for which is optimal and a further isolate the clearance data for which is optimal, compared to the, or each, other of the isolates, respectively, is then selected for use in the vaccine. There is also described a method for providing an oral killed vaccine against abnormal microbial colonisation of a mucosal surface, comprising evaluating the capacity of a plurality of different isolates of a microbe to induce expression of IL-10 and IL-12 in antigen responsive cells. At least one isolate is selected that induces optimal expression of IL-12 relative to IL-10 compared to the, or each, other of the isolates, respectively, for use in the vaccine.
Owner:HUNTER IMMUNOLOGY LTD
Method for detecting NSPs residues in foot-and-mouth disease inactivated antigens or inactivated vaccines
InactiveCN111239393AHigh sensitivityEasy to operateBiological testingImmunoassaysDiseaseElectrophoreses
The invention relates to a method for detecting NSPs residues in foot-and-mouth disease inactivated antigens or inactivated vaccines, and belongs to the technical field of immunology. The method comprises the following steps: carrying out protein electrophoresis on an inactivated antigen and a demulsified foot-and-mouth disease inactivated vaccine so as to separate proteins with different sizes inthe antigen or the vaccine, then carrying out WB, and judging whether the antigen or the vaccine contains NSPs and components of the NSPs according to an appeared band and standard contrast. The method has the advantages of strong specificity, high sensitivity and simple operation, can be operated in a common laboratory, is very suitable for foot-and-mouth disease manufacturers, and provides technical guidance for the foot-and-mouth disease manufacturers to purify antigens and formulate standardized processes.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Mycoplasma gallisepticum antibody detection reagent, preparation method and application thereof
InactiveCN111707822AIn line with the domestic marketProductiveImmunoassaysMycoplasma antibodyMethyl violet
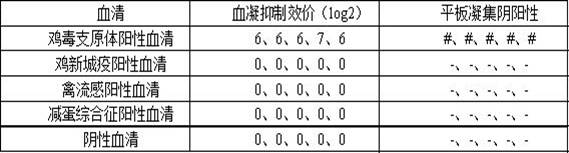
The invention relates to the technical field of poultry antibody detection reagents, particularly to a mycoplasma gallisepticum antibody detection reagent, a preparation method and application thereof. According to the invention, a mycoplasma gallisepticum culture solution is subjected to a series of treatment on, inactivating is performed, the inactivated antigen bacteria solution and a freeze-drying protective agent are uniformly mixed, and freeze drying is performed to obtain the mycoplasma gallisepticum antibody detection reagent; the reagent can be directly used for a hemagglutination inhibition test; a plate agglutination test can be carried out by adding any one coloring agent of methyl violet, amber red and crystal violet into the reagent containing 4-8HA unit of antigen; the hemagglutination value of the detection reagent reaches 26; and the detection reagent not only improves the hemagglutination titer of the mycoplasma gallisepticum antigen, but also has two purposes, is stable, sensitive, strong in specificity, long in storage life, simple to operate, convenient and fast, does not need special equipment and instruments, and can be used for efficacy test, epidemiologicalinvestigation, clinical sample detection and the like of mycoplasma gallisepticum vaccines.
Owner:兆丰华生物科技(南京)有限公司
In-situ vaccine and preparation method thereof
PendingCN113769075AIncrease profitImprove securityMaterial nanotechnologyNanomedicineAdjuvantTGE VACCINE
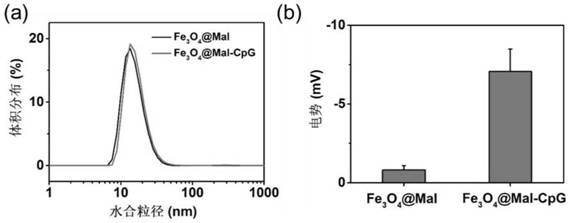
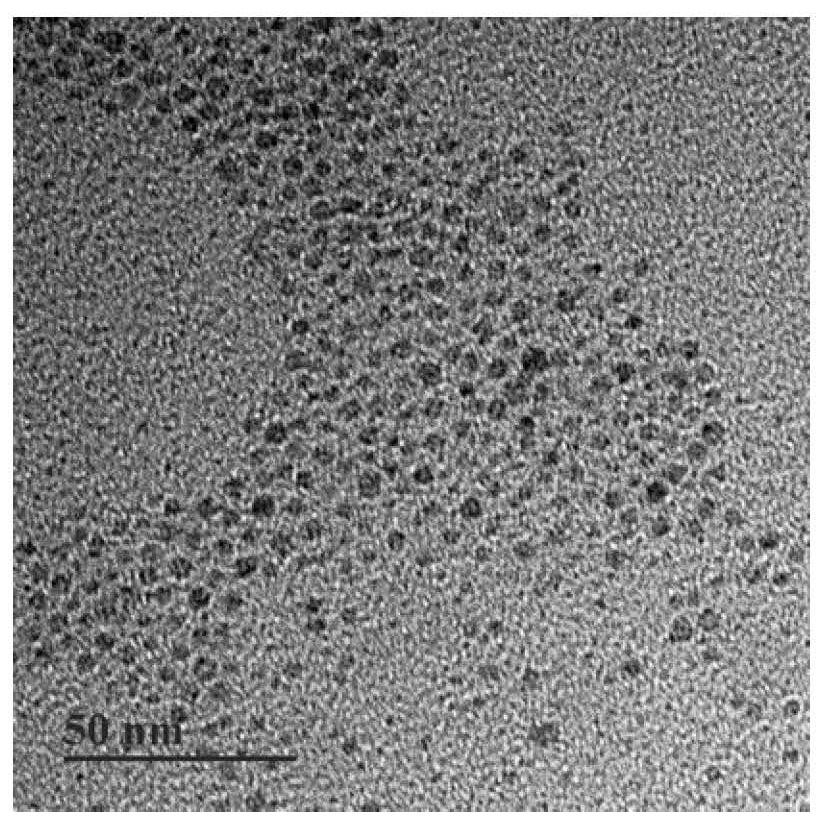
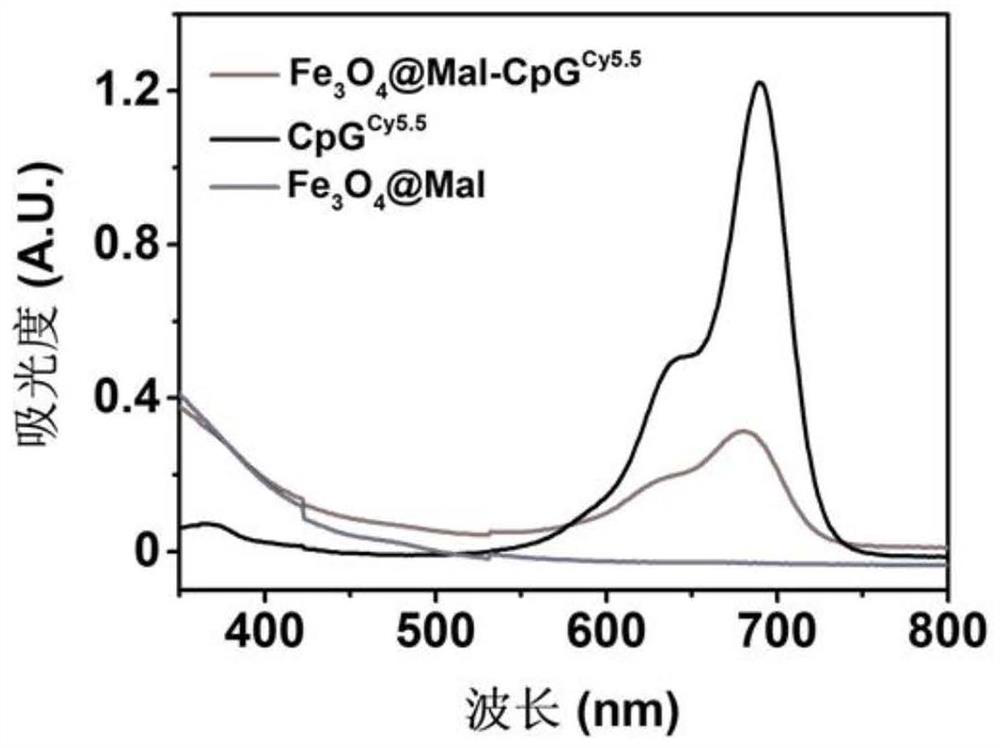
The invention relates to an in-situ vaccine and a preparation method thereof. The preparation method comprises the following steps: culturing and inactivating a tumor cell antigen stock solution; preparing a nano-material (at) Mal solution; adding an adjuvant to synthesize nano-material (at) Mal-adjuvant particles; and mixing and incubating the tumor cell antigen stock solution and the nano-material (at) Mal-adjuvant particles in a volume ratio of (5: 1)-(5: 3) to obtain a nano-material (at) Mal-adjuvant (at) antigen. The preparation method of the in-situ vaccine is environment-friendly, simple and easy to operate, and is suitable for various tumors and has universality. The maleimide on the surface of the nano-material (at) Mal can form a stable thioether bond with sulfydryl on protein so as to capture tumor-associated antigens, so that the utilization rate of the antigens is improved, and co-delivery of the adjuvant and the antigens can be realized. The nano material (at) Mal can be used as an adjuvant to activate antigen presenting cells and enhance the phagocytosis, so that the antigen capabilty is represented, T cells are further activated, and the safety and curative effect of cancer immunotherapy are improved.
Owner:SUZHOU UNIV
Composition, Preparation Method And Evaluation Of A Complex Immunogen Named I-SPGA For Production Of Immunological Active Proteins (IAP)
ActiveUS20210121552A1Increased antibody coupling powerEasy to combineBacterial antigen ingredientsEgg immunoglobulinsPotentiatorResistant infection
The present invention relates to the composition and method of preparing an immunogen designated as I-spga consisting of a complex antigen prepared from 18 to 26 species of pathogenic microorganisms isolated from patients, inactivated with binary ethyleneamine (BEI) and formalin, diluted in a SPGA immunopotentiator mixed with QS-21 adjuvant. By inoculating the hens with the I-spga immunogen, hyperimmune eggs (Immunospga) are obtained which contain immunologically active proteins specific to the 18-26 antigens used for immunization. The immune response of the hens is specific to the used antigens by amplification of the antigenic signal by the SPGA immunopotentiator and due to a special immunization program that allows the immune system to act complex and intense: The I-spga complex antigen contains 18-26 microorganisms isolated from patients, bacterial bodies, components from bodies obtained by ultrasonography, cilia, exotoxins, endotoxins, spores, viruses, fungi or yeasts. This pathogenic material is inactivated with BEI and formalin. The I-spga antigen is of three types. The standard I-spga antigen is composed of 18 to 24 antibiotic-resistant bacterial species isolated from patients in Romania. The specific I-spga complex antigen is composed of the I-spga complex antigen containing a mixture of 7-9 strains from a single species of bacteria, fungi or yeasts isolated from patients in Romania mixed with SPGA and QS-21, used for inoculation of hens previously immunized with standard I-spga antigen. The personalized I-spga antigen is composed of patient-derived pathological material containing cellular debris and pathogenic germs inactivated with BEI and formalin and mixed with SPGA and QS-21 and is used to immunize hens previously immunized with the standard I-spga antigen. This now patented technology encompasses a new generation of biological products in which the immune response of the hens to different groups of parenterally inoculated antigens at different time intervals is overlapping. Chicken response is uniform and additional administration of immunogens and SPGA as an immunopotentiator amplifies the antigenic signal and immune response. The I-spga immunogen as well as the immune response contain two markers, G and A, which identify the I-spga antigen used for immunization against the antigens used to produce the Imunoinstant group bio-preparations or similar products. The I-spga immunogen is used to immunize the hens for obtaining immunologically active proteins that can be used to treat immune deficiencies, psoriasis, epidermolysis bullosa, other dermatitises, nosocomial infections, antibiotic-resistant infections in the urinary system of children and grownups.
Owner:FANTANA RAUL SORIN +1
A kind of immunopotentiator and its application
ActiveCN108117583BEnhance immune responseGood immune effectPeptide/protein ingredientsViral antigen ingredientsCtl epitopeChemical synthesis
The invention provides an immune enhancer, a porcine foot-and-mouth disease vaccine composition containing the immune enhancer and its application. The immune enhancer includes three porcine foot-and-mouth disease virus CTL epitope polypeptides, which are one of the polypeptide shown in sequence 1 in the sequence listing, the polypeptide shown in sequence 2 in the sequence listing and the polypeptide shown in sequence 3 in the sequence listing Or any combination of two or more. The porcine foot-and-mouth disease vaccine composition comprises an immune enhancer and a porcine foot-and-mouth disease antigen. The immunopotentiator of the present invention can be prepared in large quantities by a solid-phase chemical synthesis method, and mixed with the inactivated antigen at a certain ratio, which is convenient for large-scale production and preparation of a vaccine composition, which can induce the body to produce a humoral immune response level , can also enhance the body's cellular immune response level, and can obtain a better immune effect. The immunopotentiator of the invention is safe and reliable, and has broad application prospects.
Owner:CHINA ANIMAL HUSBANDRY IND
Coating antigen, enzyme-labeled antibody and blocking method kit for detecting porcine pseudorabies virus ge antibody
ActiveCN109959788BHigh sensitivityStrong specificityBiological material analysisRabies virus strainPig breeding
The invention relates to a blocking method kit for detecting porcine pseudorabies virus gE antibody, which contains a support medium coated with the porcine pseudorabies virus mutant strain whole virus inactivated antigen of the present invention or coated pig The support medium for the whole virus inactivated antigen of the variant strain of pseudorabies virus and the inactivated antigen of the whole virus of the classic strain of pseudorabies virus, the enzyme-labeled monoclonal antibody 11H1, and the antigen-antibody reaction for the porcine pseudorabies virus Detection reagents, negative control, positive control for detection. The kit of the invention can broadly detect the gE antibody in the serum of pigs infected with porcine pseudorabies virus, has high detection sensitivity and good specificity, has few gray areas after detection, i.e. suspicious samples, and is easy to purify pseudorabies.
Owner:LUOYANG PULIKE WANTAI BIOTECH
Quantitative detection method of rabies virus inactivated antigen
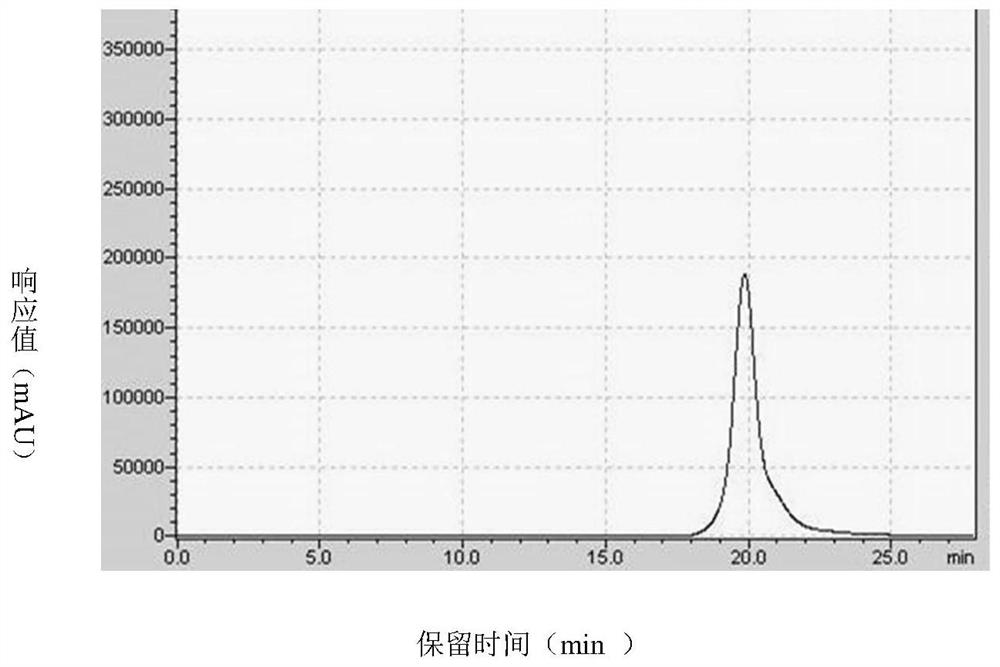
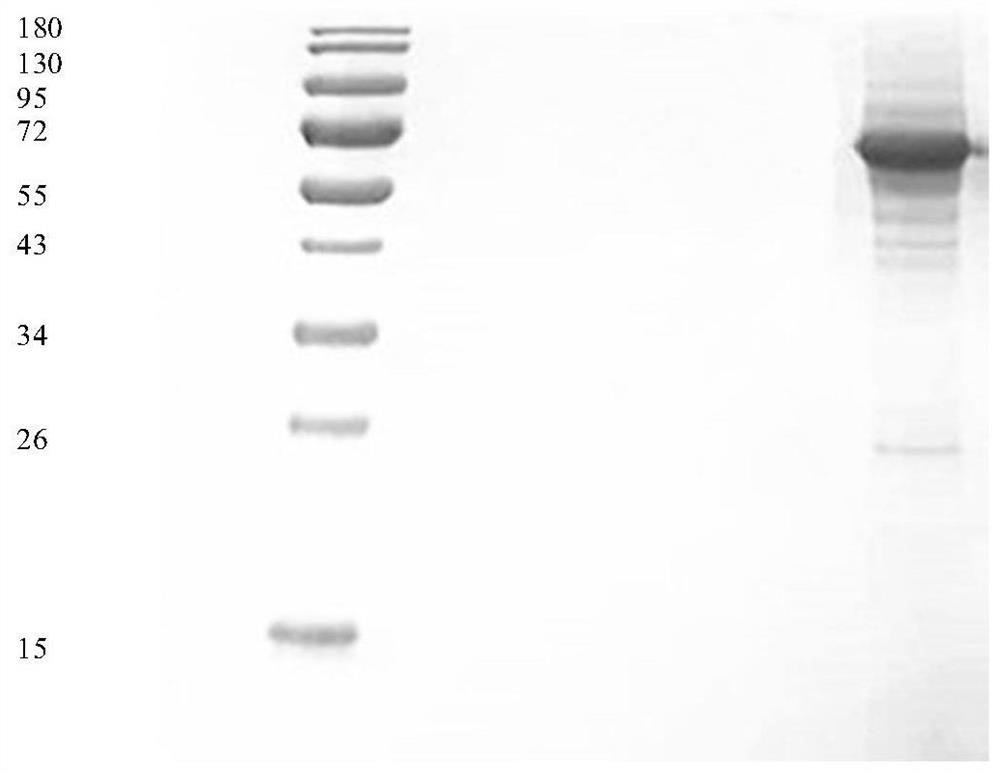
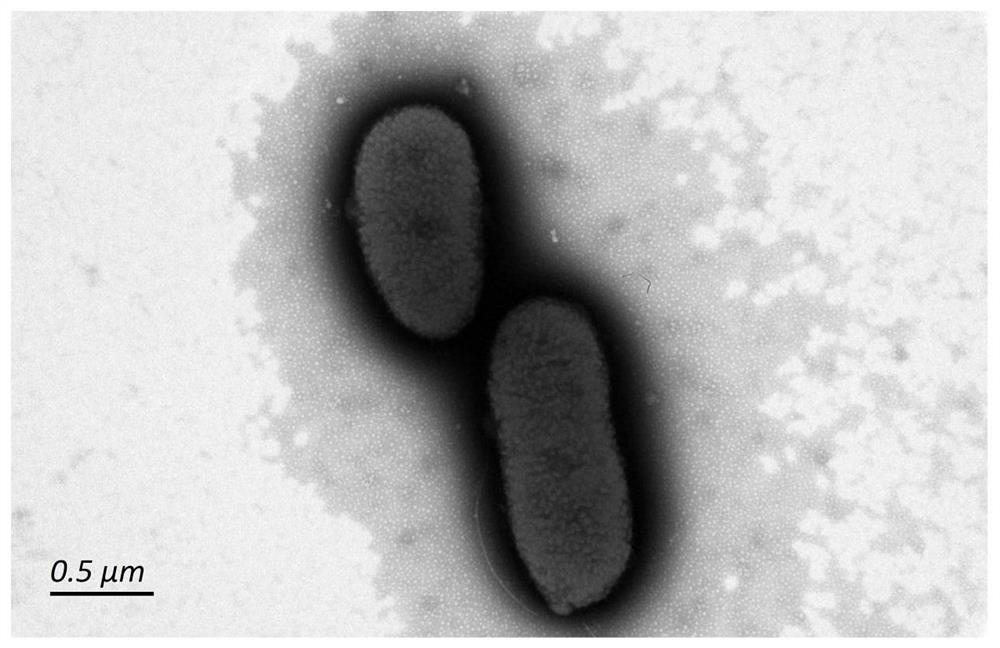
ActiveCN110274982BEfficient separationProduce Effective GuidanceComponent separationAntigenVirus inactivation
The invention discloses a quantitative detection method of rabies virus inactivated antigen. The method comprises: at first using the SDS-PAGE method and the TEM method to qualitatively identify the target peak; then using a protein quantification kit to measure the concentration of the standard substance; serially diluting the standard substance, and detecting it on a chromatograph with a gel chromatographic column According to the absorption peak of the standard substance at 280nm, the linear regression equation of concentration and peak area is established; the concentration of rabies virus inactivated antigen in the test sample is calculated according to the linear regression equation and the peak area of the test sample. The method of the invention can be used for the quality control of rabies virus production, has the advantages of rapidity, stability and good repeatability, can guide the production of vaccines, and plays an important role in improving the quality of vaccines.
Owner:CHINA ANIMAL HUSBANDRY IND
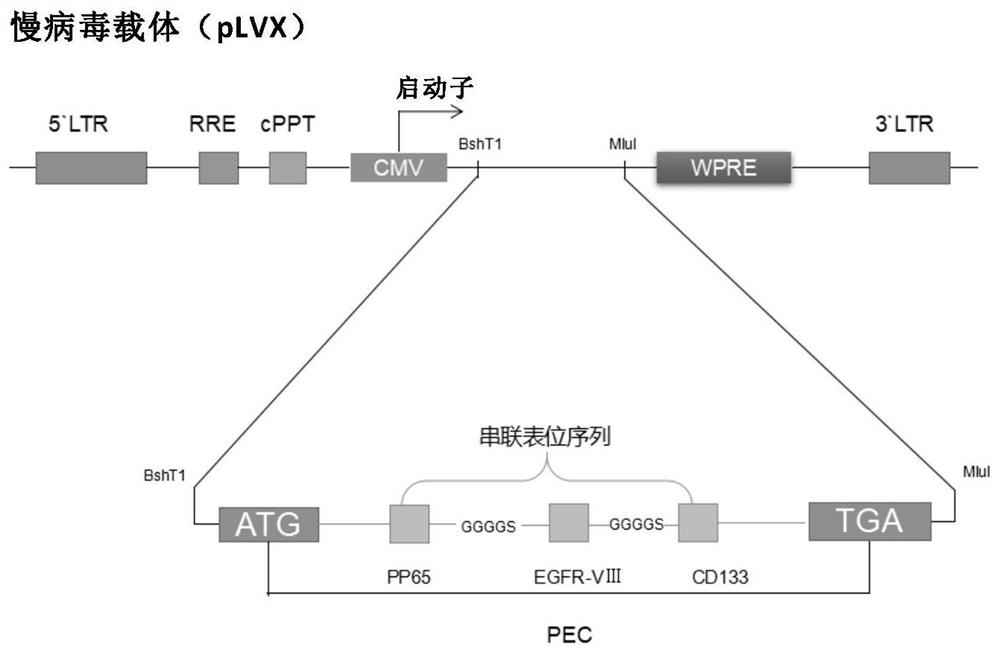
A preparation method of CTL targeting multiple epitopes of glioblastoma
The invention relates to the field of biotechnology development and application research. Specifically, the present invention provides a method for preparing cytotoxic T lymphocytes (CTL) targeting multiple glioblastoma antigen epitopes. In the method of the present invention, a new artificially encoded antigen sequence synthesized by tandem combination of three glioma-associated antigen (GAA) epitope sequences is cloned into a lentiviral expression vector, and the virus is packaged and transfected into dendritic cells, and then separated from Chosen CD3 + T cells are co-cultured to stimulate the proliferation and differentiation of antigen-specific T cells, so that the prepared CTLs have the specificity of targeting the three GAA antigens. The GAA antigen-specific CTL prepared by the invention has the advantages of strong targeting specificity, no side effects, and is not easy to cause immune escape, and has great application prospects in the treatment of GAA-expressing glioblastoma.
Owner:上海尚泰生物技术有限公司
Inactivated rabies antigen and preparation method thereof
ActiveCN102205119BProtection attackReduce production processAntiviralsAntibody medical ingredientsAntigenPropiolactone
The invention discloses an inactivated rabies antigen. A method for preparing the antigen comprises the following steps: mixing a rabies virus dG strain virus liquid with a titer of 5.0*10<6> to 1.0*10<7> FFU / mL and a BHK-21 cell suspension with a concentration of 5.0%*10<5> to 1.0*10<6> individuals / mL; cultivating for 72 to 96 h in the environment with a temperature of 33 to 37 DEG C; carrying out freeze thawing and centrifugation, and collecting a supernatant; regulating the pH of the supernatant to 7.4 to 7.6; adding beta-propiolactone with a final concentration of 0.025 to 0.05 V / V% drop by drop; and inactivating and hydrolyzing. According to the present invention, the method has the advantages of needing no condensation, simplifying production technology and saving production cost, and prepared inactivated vaccines can induce the generation of a high level of antibodies in a dog and can effectively protect the dog from virulent attack by the rabies virus. The inactivated rabies antigen prepared through the method allows condensation and purification to be omitted, production technology to be greatly simplified and production cost to be reduced, and is suitable for popularization and application in China.
Owner:GUANGZHOU SOUTH CHINA BIOLOGICAL MEDICINE
Recombinant gene VII type Newcastle disease virus strain and vaccine composition, preparation method and application thereof
ActiveCN110713987AHigh titerHigh HA titerSsRNA viruses negative-senseViral antigen ingredientsAntigenNewcastle disease virus NDV
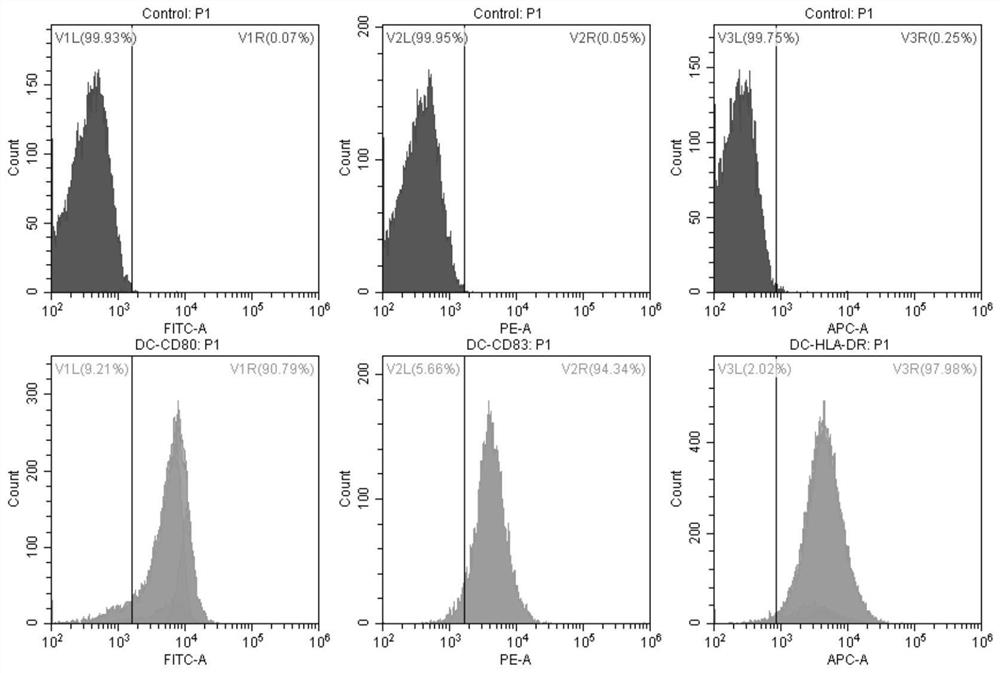
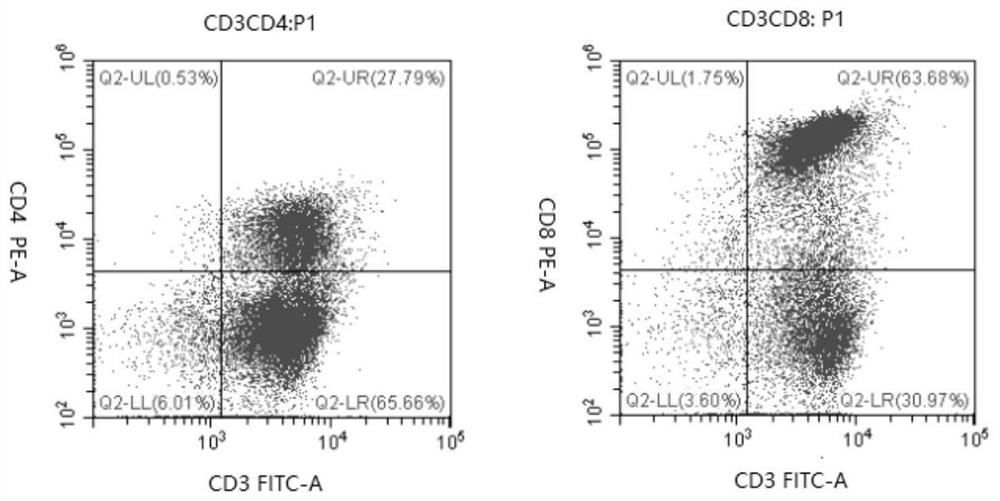
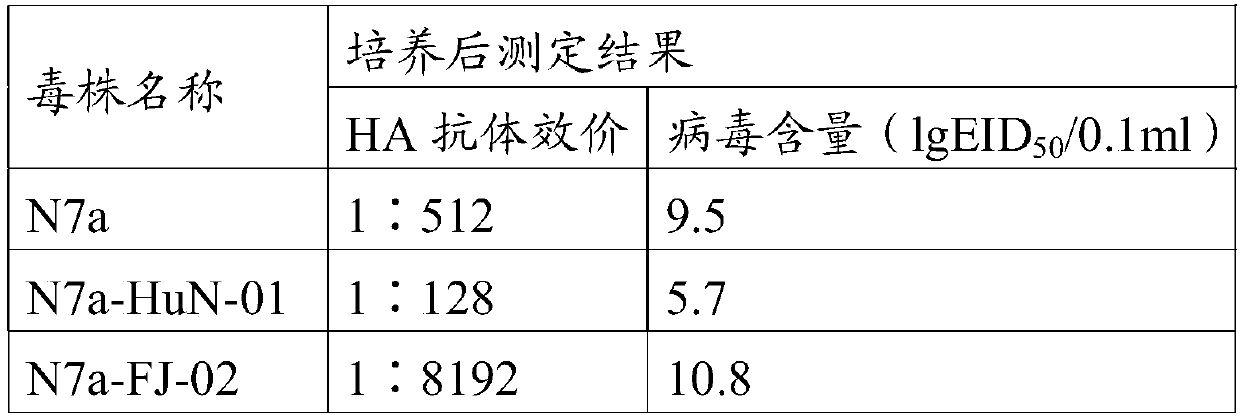
The invention discloses a recombinant gene VII type Newcastle disease virus attenuated strain rN7a strain, and further discloses a vaccine composition containing the rN7a strain or a culture-inactivated antigen of the rN7a strain. The rN7a strain is the attenuated strain obtained by replacing a P protein gene sequence of a Newcastle disease virus N7a strain with the preservation number of CCTCC NO:V201545 with a P protein gene sequence as shown in SEQ ID No.1. The rN7a strain has high virus titer and high HA titer after culture. The vaccine composition can provide complete protection to a variety of strains.
Owner:LUOYANG HUIZHONG BIOTECH
Porcine rotavirus strain, vaccine composition, preparation method and application thereof
ActiveCN108220248BImproving immunogenicityFully protectedViral antigen ingredientsAntiviralsLeb antigenPorcine rotavirus
The invention relates to a porcine rotavirus strain HN03 strain. The strain has good immunogenicity, and the vaccine composition prepared from its inactivated antigen and subunit antigen can have a good protective effect on pigs. The preparation of the invention contains pigs. The method for the vaccine composition of rotavirus inactivated antigen can cultivate the virus that produces high droplet toxicity, and the antigen content is high, thereby ensuring the immune efficacy of the vaccine.
Owner:PU LIKE BIO ENG
Triple inactivated vaccine for preventing and treating duck circovirus disease, novel duck reovirus disease and duck adenovirus type 3 and preparation method of triple inactivated vaccine
PendingCN114209821AHigh titer contentAvoid adverse reactionsViral antigen ingredientsVirus peptidesDiseaseReovirus (antigen)
The invention discloses a triple inactivated vaccine for preventing and treating duck circovirus disease, novel duck reovirus disease and duck adenovirus type 3 and a preparation method of the triple inactivated vaccine. The triple inactivated vaccine comprises an effective amount of inactivated antigen and an immunologic adjuvant in immunization, the inactivated antigen comprises a duck circovirus antigen, a novel duck reovirus antigen and a duck adenovirus type 3 antigen. The invention further discloses a method for preparing the triple inactivated vaccine. The 3-type triple inactivated vaccine for preventing and treating the duck circovirus disease, the novel duck reovirus disease and the duck adenovirus disease is good in safety, and mutual interference or influence of antigen components cannot be generated. Immune protection efficacy tests prove that the triple inactivated vaccine can prevent duck reovirus, duck circovirus and duck adenovirus type 3 at the same time, can avoid adverse reactions caused by multiple inoculation immunization, and has the advantages of simple preparation method, convenience, multiple effects, low cost, high vaccine titer content and the like.
Owner:哈药集团生物疫苗有限公司
A kind of foot-and-mouth disease virus competition ELISA detection kit
The invention relates to a competitive ELISA detection kit for foot-and-mouth disease virus antibody, the kit comprising: single domain antibody sdAb-A; A / AKT-III strain FMDV inactivated antigen; monoclonal antibody 9C10, blocking solution; gold-labeled secondary antibody ; and supporting media. By combining the FMD antigen with the specific single-domain antibody coated on the support medium, the monoclonal antibody 9C10 and the antibody in the serum to be tested compete with the antigen, and it is detected by gold-labeled horse anti-mouse IgG, when the sample is detected It only needs to add the sample to be tested and incubate with monoclonal antibody 9C10 for 1 hour, then add the gold-labeled secondary antibody for 10 minutes of color development, and the value can be obtained after washing. The operation is simple and the diagnosis is fast. The competitive ELISA detection kit of the invention has high detection sensitivity, and the coincidence rate with virus neutralization reaction is higher than other commercial detection kits.
Owner:内蒙古必威安泰生物科技有限公司
Newcastle disease and H9 subtype avian influenza bivalent inactivated vaccine containing immunopotentiator as well as preparation method and application of newcastle disease and H9 subtype avian influenza bivalent inactivated vaccine
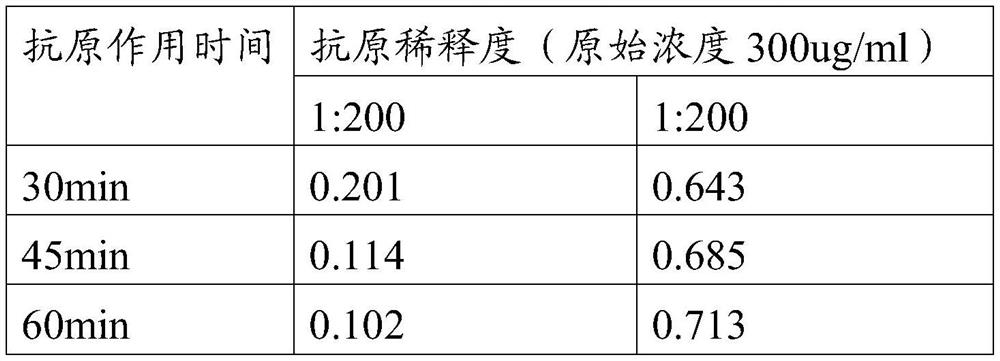
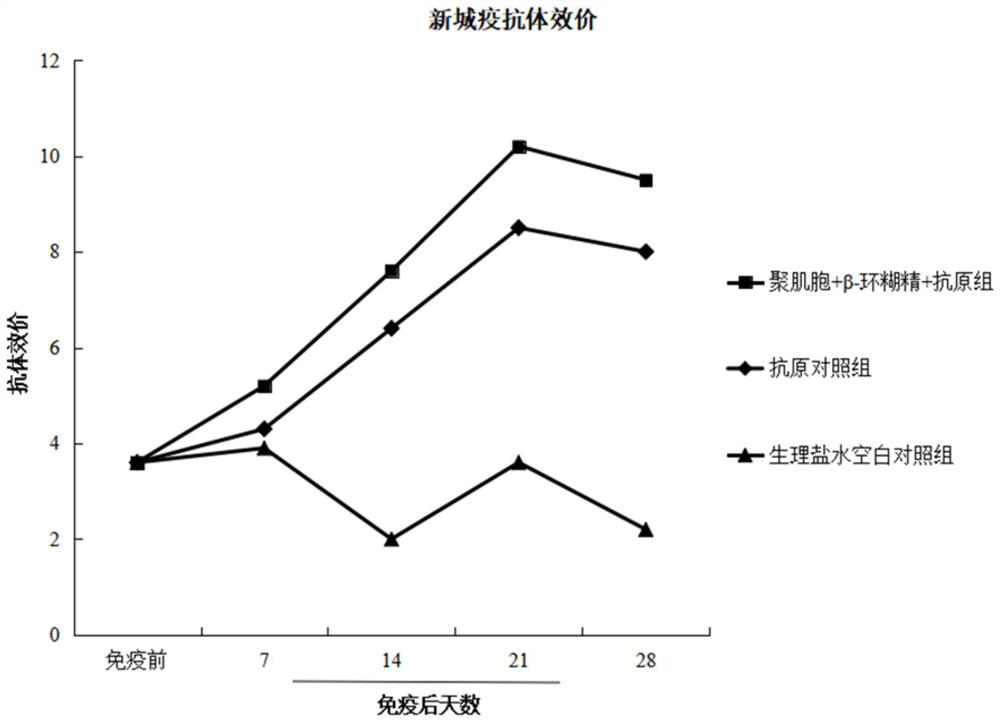
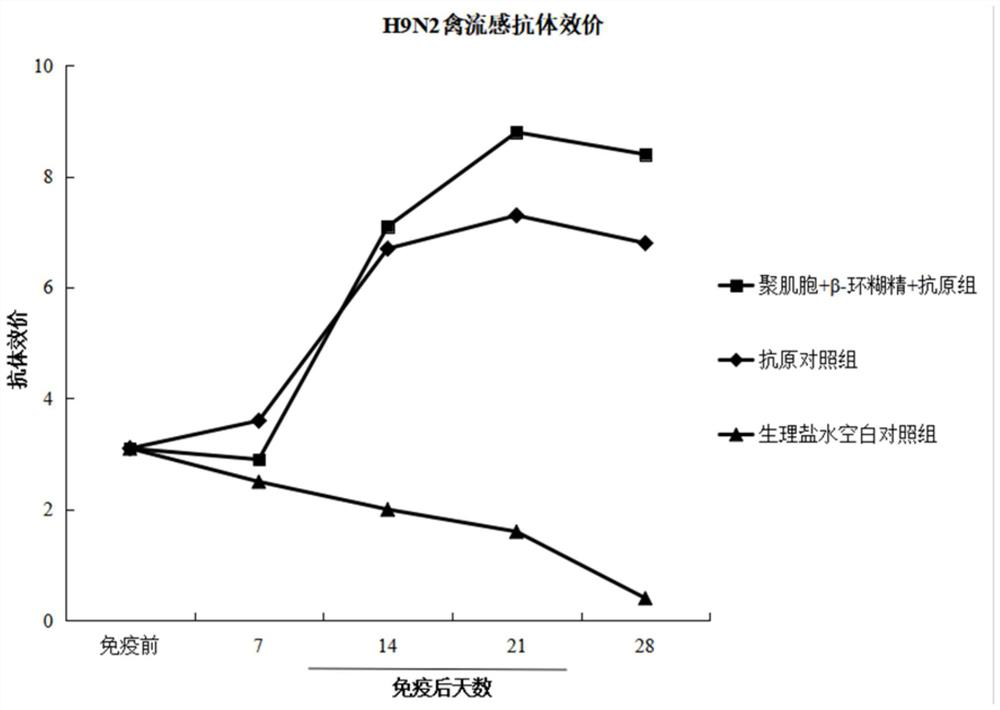
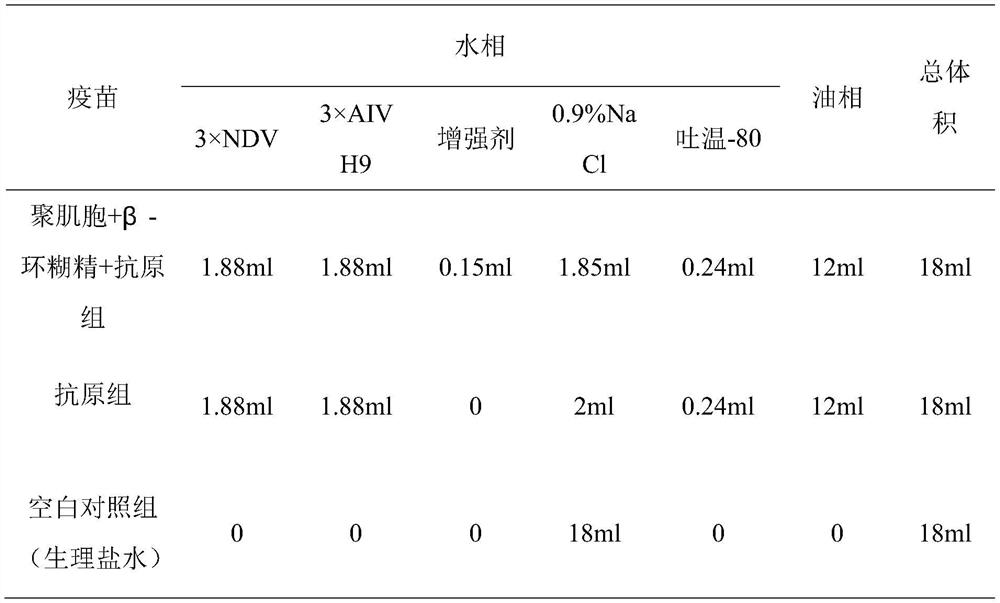
PendingCN114288403AControlled release rateImprove bioavailabilityViral antigen ingredientsAntiviralsCyclodextrinAluminum Stearate
The invention discloses a newcastle disease and H9 subtype avian influenza bivalent inactivated vaccine containing an immunopotentiator as well as a preparation method and application of the newcastle disease and H9 subtype avian influenza bivalent inactivated vaccine, the immunopotentiator contains 1-1000 [mu] g / ml of polyinosinic acid and 1-1000 [mu] g / ml of beta-cyclodextrin, and the immunopotentiator, an inactivated antigen and Tween are mixed to prepare a water-phase solution; mixing white oil, span and aluminum stearate to obtain an oil phase solution; and mixing the oil-phase solution with the water-phase solution to obtain the inactivated vaccine. The immunopotentiator contained in the inactivated vaccine and other components in the vaccine have positive guidance quality; the preparation has the advantages of increasing the solubility, dissolution rate and stability of vaccine components, controlling the release rate of the vaccine, improving the bioavailability of the vaccine, reducing toxic and side effects, starting an immune response program contained in an organism and the like, so that the antibody level of the immunized organism is improved, and the immune effect is enhanced; after immunization, the chicken antibody response level is obviously enhanced, the capability of generating high antibodies is achieved, the immune effect is good, and the immune protection acting force is lasting and long.
Owner:浙江洪晟生物科技股份有限公司 +2
Method for determining titer of swine flu inactivated vaccine
ActiveCN102735853BReduced measurement timeReduce operating errorsBiological testingRed blood cellPorcine influenza
The invention discloses a method for determining titer of a swine flu inactivated vaccine. The method comprises the following steps: immunizing a pig with a swine flue inactivated vaccine, collecting blood 21-28 days after immunization and separating blood serum; removing non-specific components in the serum to obtain a serum to be tested; diluting the swine flue inactivated antigen, and preparing a unit swine flue antigen diluent with a concentration of 4HA; and diluting the serum to be tested by multiple proportions, successively adding the 4HA unit swine flue antigen diluent and 1% red cells to conduct hemagglutination inhibition test, so as to completely inhibit highest dilution of the 4HA unit swine flu antigen serum at HI titer. The method has advantages of greatly shortened measurement time, little operation error, strong controllability, and small inter-batch difference, and also reduces detection errors caused by different levels of experimental animals in an animal challenge protection experiment for testing the titer.
Owner:GUANGZHOU SOUTH CHINA BIOLOGICAL MEDICINE +1
Preparation method of bluetongue virus bivalent inactivated vaccine
PendingCN112870346AImprove immune efficiencyImprove immunityViral antigen ingredientsInactivation/attenuationAntigenAdjuvant
The invention belongs to the technical field of research and development of biological product preparation processes, and discloses a preparation method of a bluetongue virus bivalent inactivated vaccine, which comprises the steps of virus culture, inactivator preparation, inactivation operation, concentration and purification, antigen emulsification, vaccine proportioning and the like. According to the invention, the adjuvant, the excipient and the inactivated antigen suspension are combined, the ruminant is efficiently induced, the immune response to the bluetongue virus is generated, the interference of the non-structural protein is effectively removed, and the distinguishable identification of the vaccine immunity and the wild strain infection is achieved.
Owner:YUNNAN ANIMAL SCI & VETERINARY INST
Veterinary vaccine diluent with immunological enhancement function as well as preparation method and application of veterinary vaccine diluent
PendingCN114306590AHigh activityIncrease proliferative activityInorganic non-active ingredientsPharmaceutical delivery mechanismAntigenMicrofiltration membrane
The invention discloses a veterinary vaccine diluent with an immunological enhancement effect, which comprises dendrobium officinale polysaccharide and normal saline, and m: v is (1: 10000)-(1: 2). The preparation process comprises the following steps: weighing the dendrobium officinale polysaccharide and the normal saline according to the mass volume ratio; and dissolving the dendrobium officinale polysaccharide in normal saline, fixing the volume, and filtering and sterilizing by using a sterile microfiltration membrane to obtain the vaccine diluent. The veterinary vaccine diluent and the powder inactivated antigen which are mixed in any proportion are mixed to prepare the vaccine, and generation of antibodies is promoted.
Owner:WEST ANHUI UNIV +1
Kit for quantitatively detecting non-structural protein residues in foot-and-mouth disease inactivated antigen and application thereof
InactiveCN111273034AHigh sensitivityThe calculation result is accurateChemiluminescene/bioluminescenceBiological testingDiseaseLeb antigen
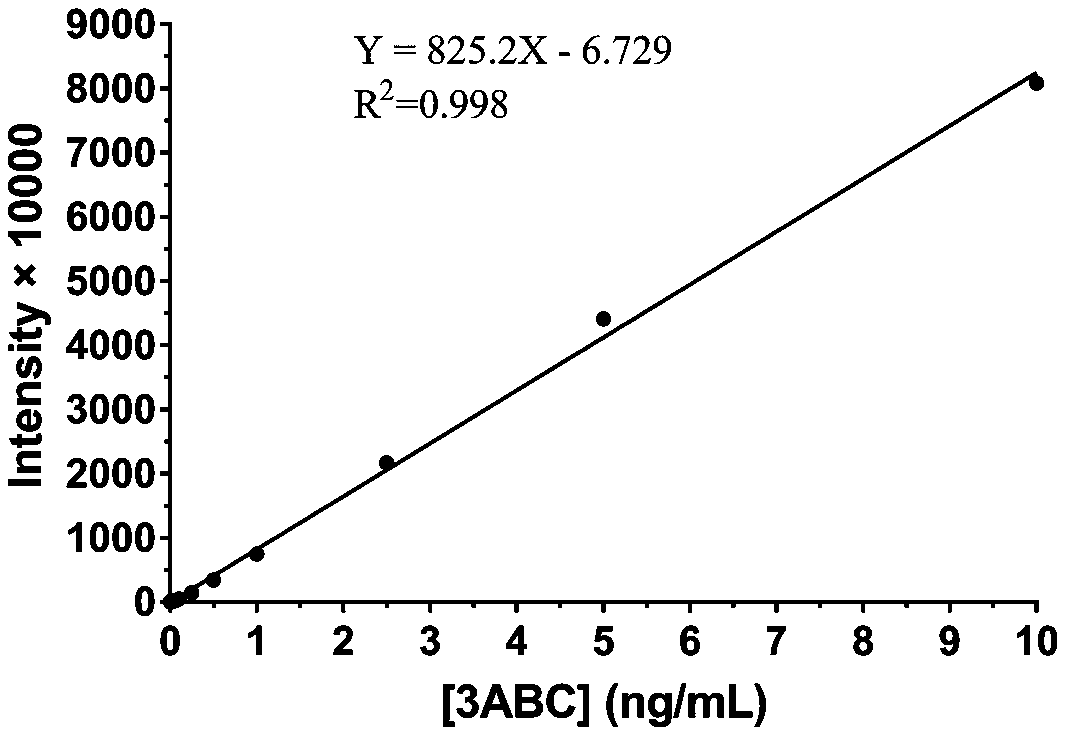
The invention relates to a chemiluminiscence kit for quantitatively detecting non-structural protein residues in foot-and-mouth disease inactivated antigens and application thereof, and belongs to thetechnical field of biology. The kit comprises a chemiluminiscence plate coated with a 3ABC polyclonal antibody, an enzyme-labeled monoclonal antibody, an antigen standard substance, a serum diluent,a 20 * PBST washing solution and a chemiluminiscence solution; the chemiluminiscence plate coated with the 3ABC polyclonal antibody is a white detachable polystyrene 96-pore plate; the enzyme-labeledmonoclonal antibody is an enzyme-labeled monoclonal antibody 9E2-HRP for recognizing 3B protein; the antigen standard substance contains 3ABC with the concentration of 10 ng / ml, BSA with the mass fraction of 0.2% and prolin 300 with the volume fraction of 0.05%. The kit disclosed by the invention is high in sensitivity, and can be used for quickly quantifying non-structural protein in the foot-and-mouth disease inactivated antigen.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Immunological adjuvant composition, preparation method and application thereof
PendingUS20220047699A1Easy to makeEffective stimulationAntibacterial agentsBacterial antigen ingredientsLeb antigenVirus antigen
The present disclosure provides an adjuvant composition containing 0.2%-15% w / v carbomer, 0.1%-0.5% w / v lecithin, and 0.03%-0.2% w / v ginsenoside. The adjuvant composition of the present disclosure cannot only ensure the long-term clarification and / or stability of the vaccine, but also can effectively stimulate the inactivated antigens and subunit antigens therein to produce high-titer antibodies for immune protection. The inactivated vaccines or subunit vaccines prepared by the adjuvant composition of the present disclosure can be used as a diluent for freeze-dried live virus antigens and has no toxic effect on the live virus antigens.
Owner:LUOYANG SEIWEI BIOTECHNOLOGIES CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com