Method for determining titer of swine flu inactivated vaccine
An inactivated vaccine and potency determination technology, which is applied in the field of veterinary biological products, can solve the problems of respiratory natural barrier damage, poor controllability, economic loss, etc., and achieve the effects of shortening the measurement time, simple and convenient measurement method, and small operational errors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0025] Below in conjunction with embodiment, further illustrate the present invention.
[0026] Obtaining and pretreatment of porcine serum:
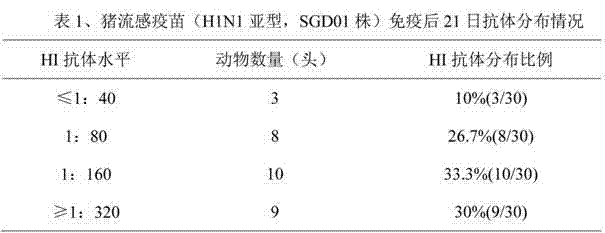
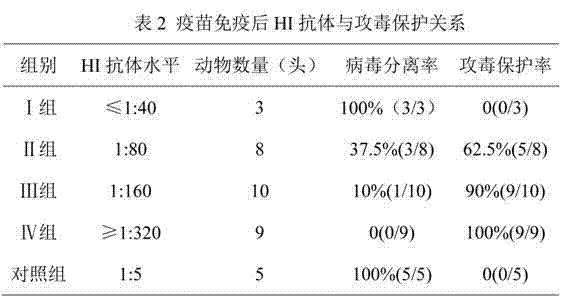
[0027] 1) Five 35-day-old negative (HI subtype influenza HI antibody titer ≤ 1:20) susceptible piglets were injected with 3 ml of swine influenza inactivated vaccine (H1N1) at two points on the left and right sides of each neck muscle. Day, together with 5 control pigs, blood was collected and serum was separated;
[0028] 2) In order to remove the non-specific components in the serum, mix the serum with the receptor-destroying enzyme (RDE) at a volume ratio of 1:4, place it in a water bath at 37°C for 16-18 hours, and then transfer it to a water bath at 56°C for 30 minutes to inactivate it Residual RDE, take it out and cool it down;
[0029] 3) Add concentrated chicken red blood cells so that the final concentration is 5% to 20%, and adsorb at room temperature for 30 minutes to remove other non-specific components;
[0030] 4) The...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com