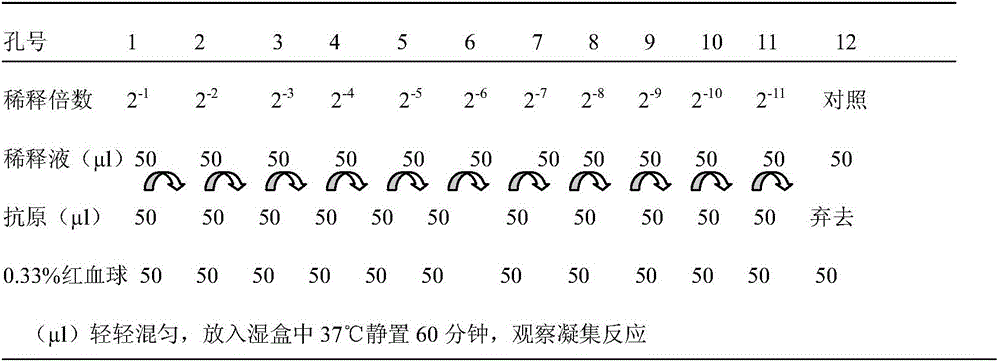
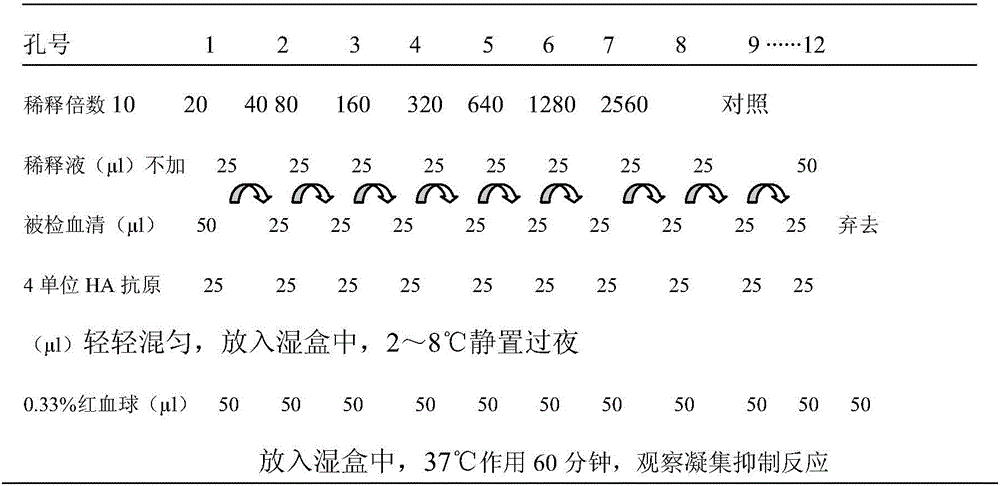
Patents
Literature
37 results about "Hemagglutination Inhibition Tests" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
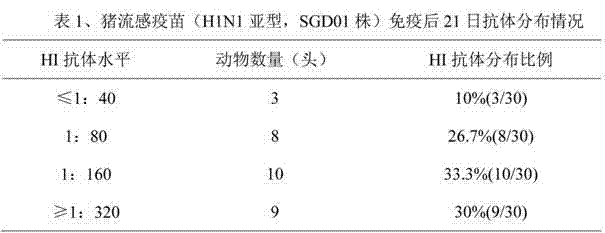
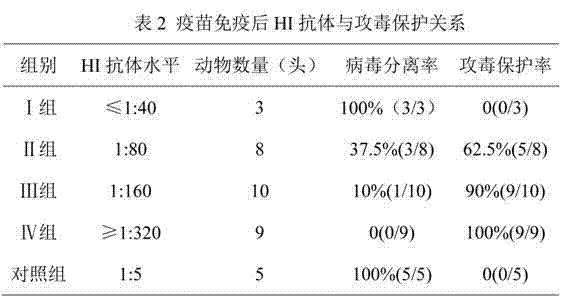
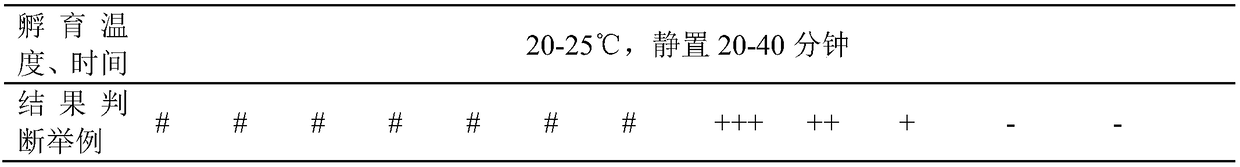
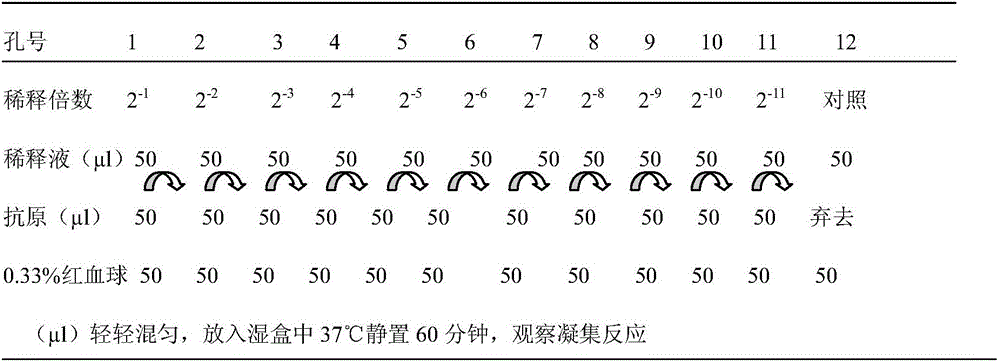
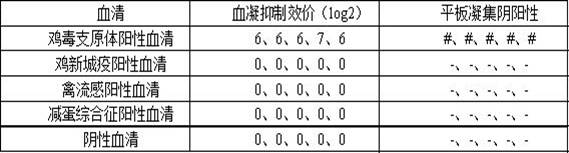
Serologic tests in which a known quantity of antigen is added to the serum prior to the addition of a red cell suspension. Reaction result is expressed as the smallest amount of antigen which causes complete inhibition of hemagglutination.
GeXP rapid detection kit for identifying eight types of porcine viral diseases and primer group thereof
ActiveCN103981286AStrong specificityHigh sensitivityMicrobiological testing/measurementMicroorganism based processesViral diseaseMultiplex pcrs
The invention discloses a GeXP rapid detection kit for identifying eight types of porcine viral diseases and a primer group thereof. Nine pairs of specific primers and a pair of universal primers are designed by the inventor based on research of a GeXP system, accordingly, a GeXP rapid detection method for identifying the eight types of porcine viral diseases is established, and the corresponding detection kit is prepared. The GeXP rapid detection method and the detection kit have the advantages of good specificity, high sensitivity, convenience, high efficiency and the like and can be used for simultaneously detecting and identifying eight types of pathogens. Compared with identification results of conventional experiment methods such as virus isolation and hemagglutination inhibition test, the coincidence rate of the GeXP rapid detection method reaches 100%. The GeXP rapid detection method and the detection kit can be used for effectively solving amplification preference in a multiplex-PCR (polymerase chain reaction) process, provide simple, convenient and high-throughput detection means for detection of main porcine common infectious diseases and pathogens thereof, meet practical requirements and have a broad application prospect.
Owner:GUANGXI VETERINARY RES INST
Method for preparing anti-dog parvovirus egg yolk antibody biological preparation
InactiveCN101062413AAvoid infectionHigh clinical cure rateImmunoglobulins against virusesAntiviralsDiseaseOil emulsion
The invention discloses a preparing method of anti-dog parvoviral vitelline antibody biological preparation, which comprises the following steps: collecting disease material of dead dog with parvoviral infection; centrifuging; removing depositing slag; centrifuging the supernatant fluid; adding into chloroform; vibrating severely; centrifuging; getting the supernatant fluid; aldehyde-washing pig red cell; adding the supernatant into the pig red cell; vibrating evenly; stewing; centrifuging; getting depositing liquid; adding into salt water; stewing; centrifuging; getting virus liberating liquid; testing HA potency with liberating liquid; reaching 1: 4096 effective; deactivating the virus liberating liquid with methyl aldehyde; adding into oil emulsion dog parvoviral vaccine prepared by brown algae polysaccharide; passing the vaccine to hen after delivery; testing HI potency of immune ovum with hemagglutination inhibition test; harvesting vitelline after reaching standard; diluting with sterile distilled water; proceeding acid treatment with lactic acid liquid; removing mixed protein; degreasing with freeze-thaw; salting-out suction liquid; adjusting IgY density; loading; putting into bathing box; treating; getting the oral biological preparation.
Owner:SHANGHAI JIAO TONG UNIV
Full-automatic Newcastle disease detector
InactiveCN105974145AFully automatedImprove sampling efficiencyMaterial analysisMedical equipmentNewcastle disease virus NDV
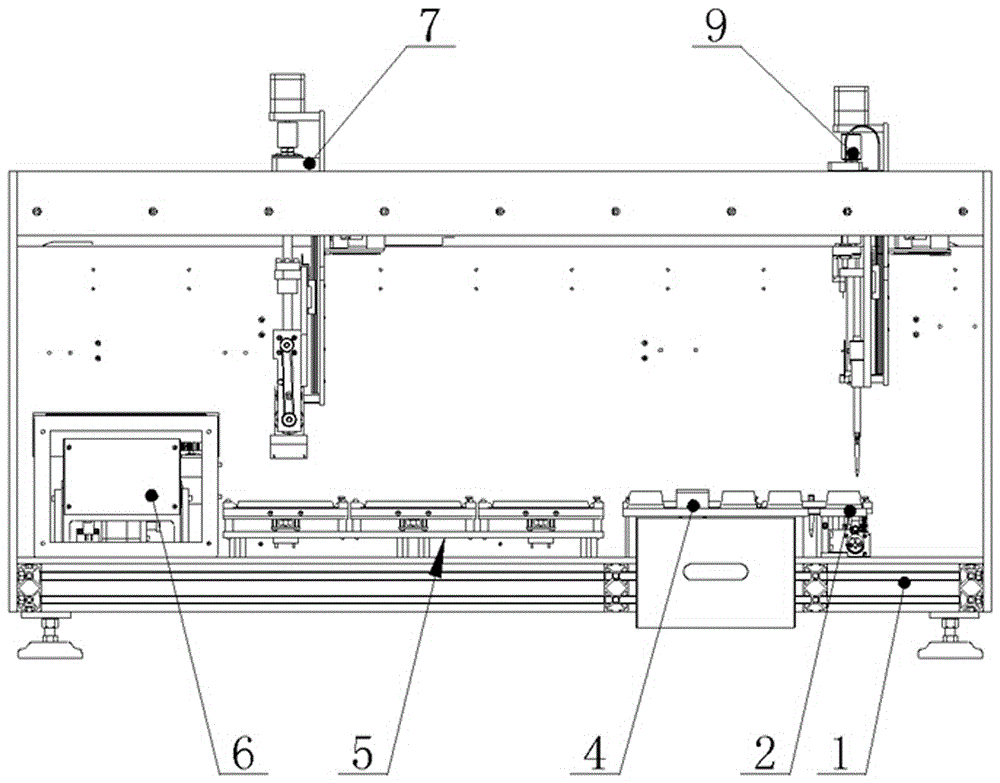
The invention discloses a full-automatic Newcastle disease detector and relates to the technical field of animal medical equipment. The full-automatic Newcastle disease detector comprises a red blood cell supply unit, a virus liquid supply unit, a reagent supply unit, a reaction plate production unit, a vision interpretation unit, an interpretation region motion unit, a suction head supply unit, a sample feeding region motion unit and a single-shaft double-sliding block motion unit, wherein all the units are mounted on a base. According to the full-automatic Newcastle disease detector, Newcastle disease blood coagulation test and Newcastle disease blood coagulation inhibition test can be simultaneously carried out on Newcastle disease virus samples. The full-automatic Newcastle disease detector is simple and compact in structure and is suitable for batch, rapid and effective tests.
Owner:SHANDONG DOLANG TECH EQUIP
Primer pair for identifying newcastle disease virus and multi-subtype avian influenza virus and application thereof
InactiveCN102321769AStrong specificityHigh sensitivityMicrobiological testing/measurementMicroorganism based processesNewcastle disease virus NDVVirus influenza
The invention discloses a primer pair for identifying newcastle disease virus and multi-subtype avian influenza virus and an application thereof. The PCR primer pair composition provided by the invention comprises primer pair NDV, primer pair H3V, primer pair H5V, and primer pair H9V; the PCR primer pair composition is applicable to the preparation of reagents for the diagnosis or auxiliary diagnosis of newcastle disease and avian influenza, or to the preparation of reagents for the identification or auxiliary identification of newcastle disease virus and multi-subtype avian influenza virus. When the PCR primer pair composition provided by the invention is used to simultaneously detect newcastle disease virus, H3, H5 and H9-subtype avian influenza virus, the specificity is strong; the sensitivity is 1EID50 / 100 microliters, 1EID50 / 100 microliters, 1*10-2EID50 / 100 microliters, and 1EID50 / 100 microliters respectively; compared with identification results of routine test methods such as virus isolation and hemagglutination inhibition tests, the results obtained by using the PCR primer pair composition of the invention has a coincidence rate of up to 100%; the PCR primer pair composition provided by the invention is applicable to the development of corresponding multiplex RT-PCR kits for the clinical diagnosis and epidemiological control of newcastle disease and avian influenza.
Owner:CHINA AGRI UNIV
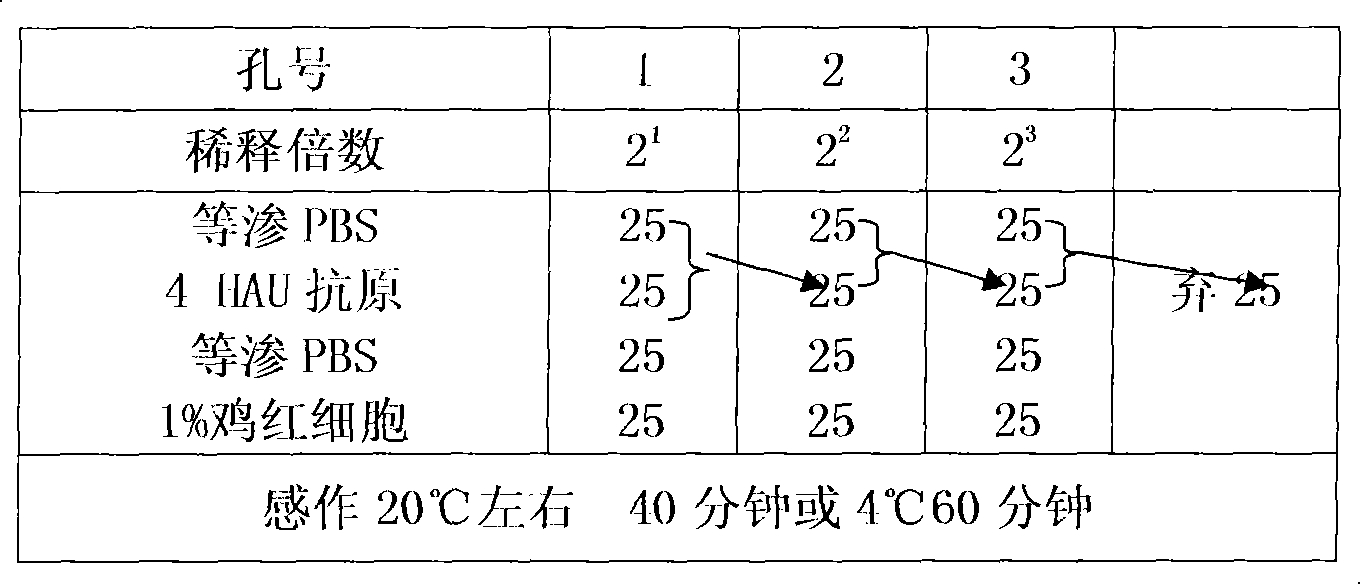
Method for detecting hemagglutination inhibition antibody of chicken infectious bronchitis
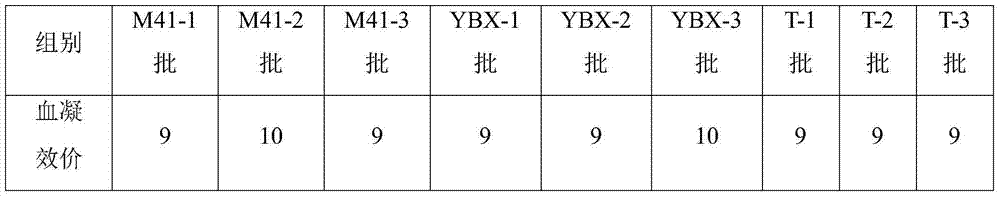
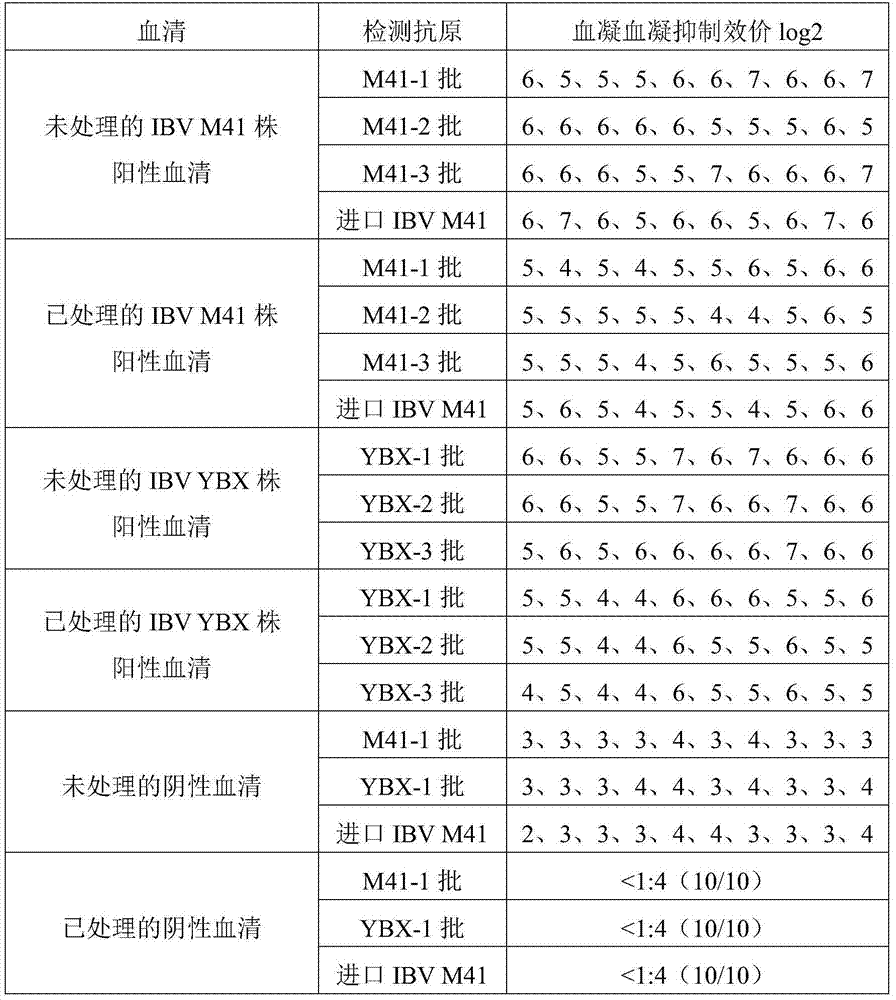
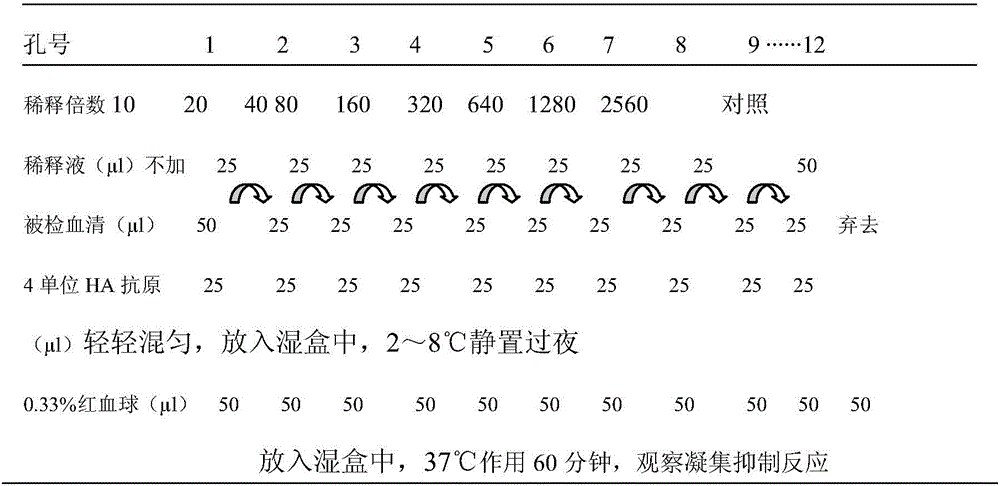
The invention relates to the technical field of livestock vaccine, and in particular relates to a method for detecting a hemagglutination inhibition antibody of chicken infectious bronchitis. The method comprises the following steps: treating serum to be detected, namely, by taking kaoline suspension as a serum treatment liquid for treating serum to be detected, adding the supernate into chicken erythrocyte blood corpuscle mud so as to obtain serum which is diluted in a ratio of 1:4 and is to be detected; performing hemagglutination inhibition test, namely, adding 25mu l of the serum which is diluted in a ratio of 1:4 and is to be detected into 25mu l of antigen with 4HA units, and performing hemagglutination judgment. By adopting the negative serum treated by using a method for removing non-specific reaction of the serum, the infectious bronchitis hemagglutination inhibition evaluation titer is less than 1:4, and by adopting the infectious bronchitis positive serum treated by using the method, the infectious bronchitis hemagglutination inhibition evaluation titer is reduced by 0.5-1 titer, the non-specificity reaction is removed, and the accuracy of the infectious bronchitis hemagglutination inhibition experiment result is improved.
Owner:YEBIO BIOENG OF QINGDAO
A rapid antigen detection method for inactivated oil emulsion vaccine against avian influenza finish products
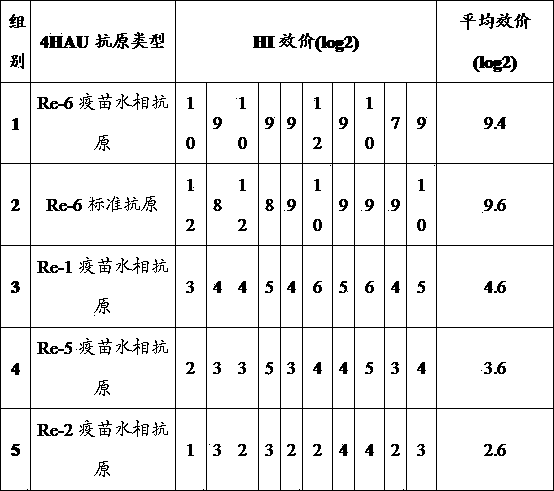
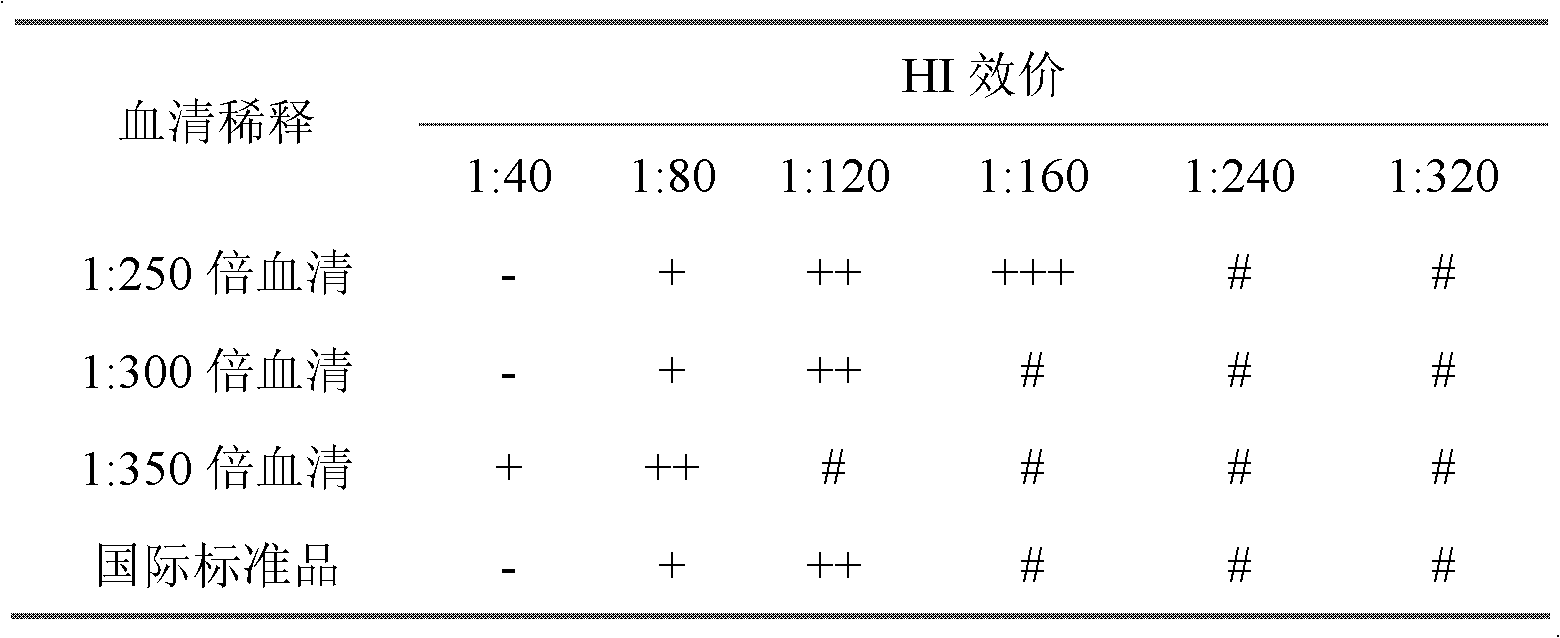
ActiveCN103235139ADoes not destroy hemagglutination titerDoes not destroy antigenicityBiological testingOil emulsionHaemagglutination inhibition
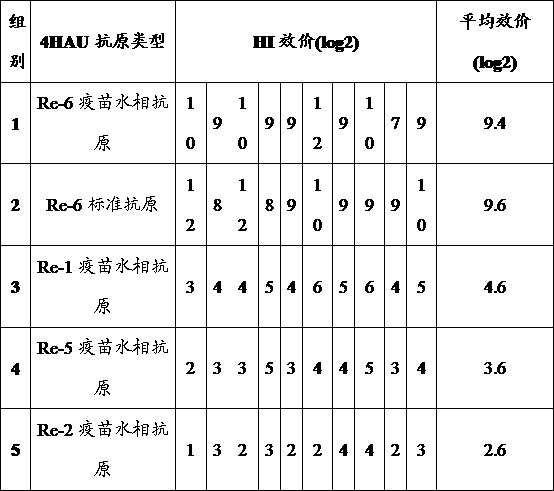
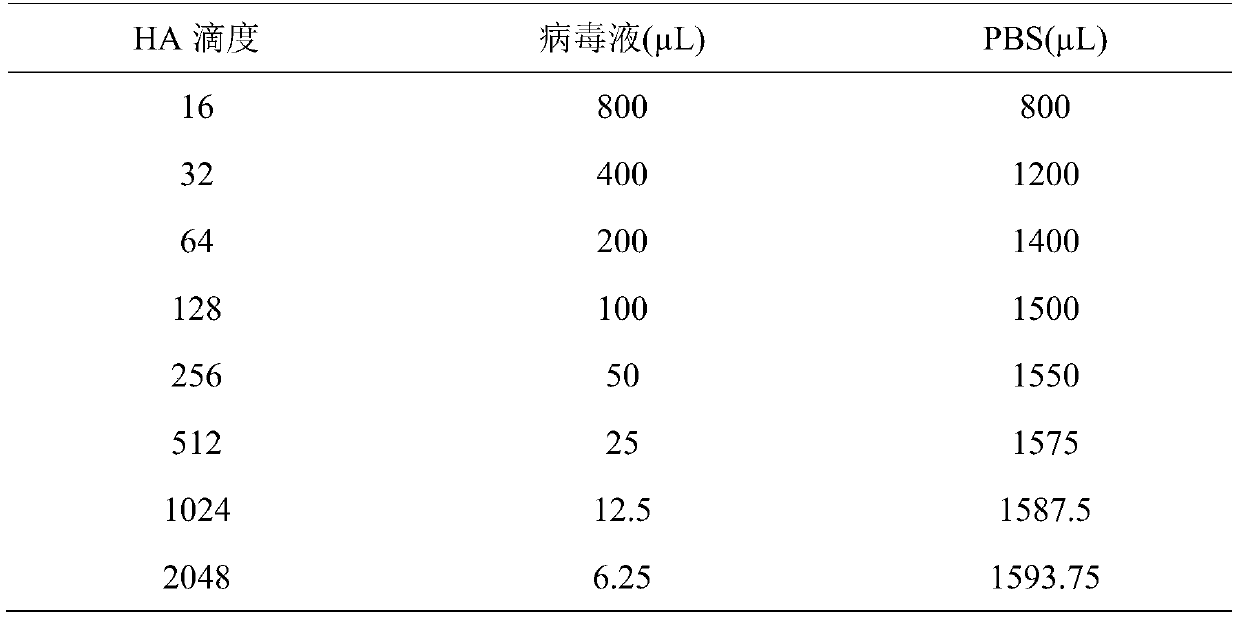
The present invention discloses a rapid antigen detection method for inactivated oil emulsion vaccine against avian influenza finish products, and the method comprises the following steps of: 1) mixing uniformly isopropyl myristate and the inactivated oil emulsion vaccine against avian influenza by thoroughly shaking, centrifuging, and separating a water phase layer; 2) performing HA titer detection to the water phase of the vaccine obtained in the step 1); 3) according to the detection result of the HA titer detection of the water phase of the vaccine in step 2), taking the water phase of the inactivated oil emulsion vaccine against avian influenza of step 1) to prepare a 4HAU vaccine antigen diluent; and 4) performing hemagglutination inhibition tests by using the 4HAU vaccine antigen diluent prepared in step 3), wherein a HI titer is expressed as the highest dilution serum that completely inhibits the 4HAU antigen. The method of the invention does not destroy the hemagglutination titer and antigenicity of vaccine antigens, can quickly and accurately determine the HI titer of the water phase of the inactivated oil emulsion vaccine against avian influenza finish products and analyze differences in antigenicity, and reagents in use are safety and non-toxic for human and environment, and are cheap and readily available.
Owner:ZHAOQING DAHUANONG BIOLOGIC PHARMA +2
Method for determining titer of swine flu inactivated vaccine
ActiveCN102735853AReduced measurement timeReduce operating errorsBiological testingRed blood cellEngineering
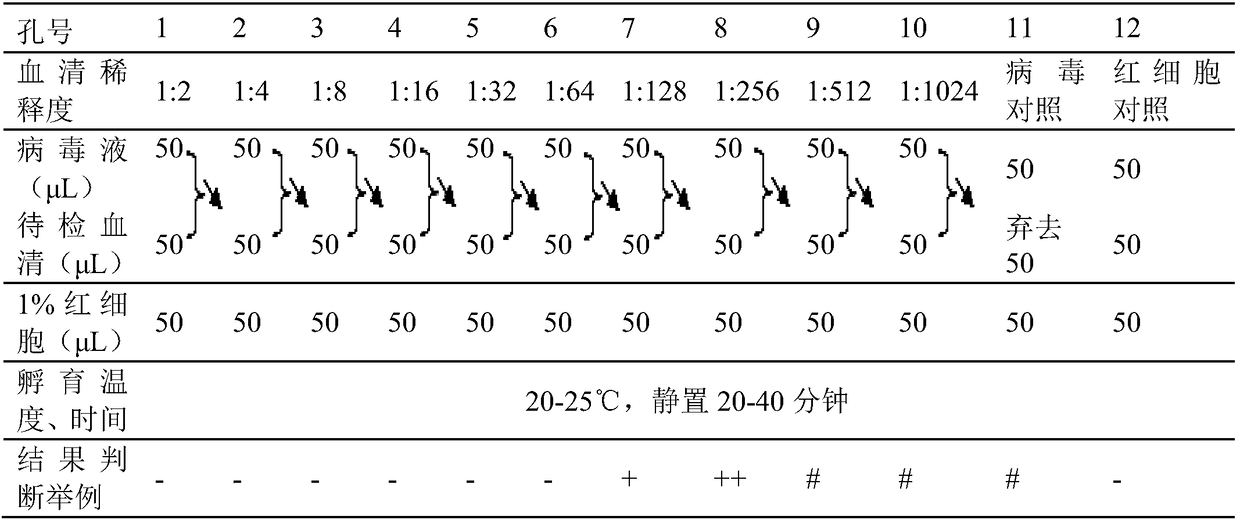
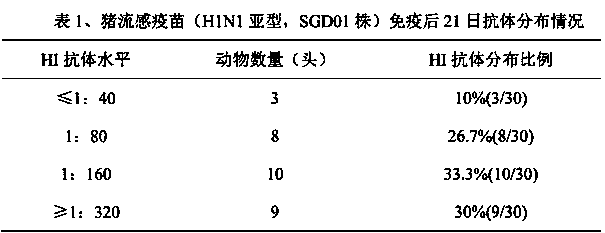
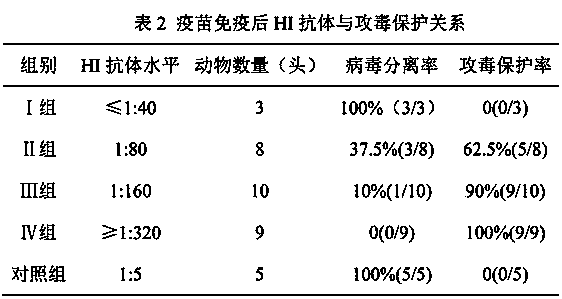
The invention discloses a method for determining titer of a swine flu inactivated vaccine. The method comprises the following steps: immunizing a pig with a swine flue inactivated vaccine, collecting blood 21-28 days after immunization and separating blood serum; removing non-specific components in the serum to obtain a serum to be tested; diluting the swine flue inactivated antigen, and preparing a unit swine flue antigen diluent with a concentration of 4HA; and diluting the serum to be tested by multiple proportions, successively adding the 4HA unit swine flue antigen diluent and 1% red cells to conduct hemagglutination inhibition test, so as to completely inhibit highest dilution of the 4HA unit swine flu antigen serum at HI titer. The method has advantages of greatly shortened measurement time, little operation error, strong controllability, and small inter-batch difference, and also reduces detection errors caused by different levels of experimental animals in an animal challenge protection experiment for testing the titer.
Owner:GUANGZHOU SOUTH CHINA BIOLOGICAL MEDICINE +1
Blight hemagglutinin detecting analysis system
InactiveCN101510235AEasy to traceImprove management abilityBiological testingSpecial data processing applicationsHemagglutininBird flu
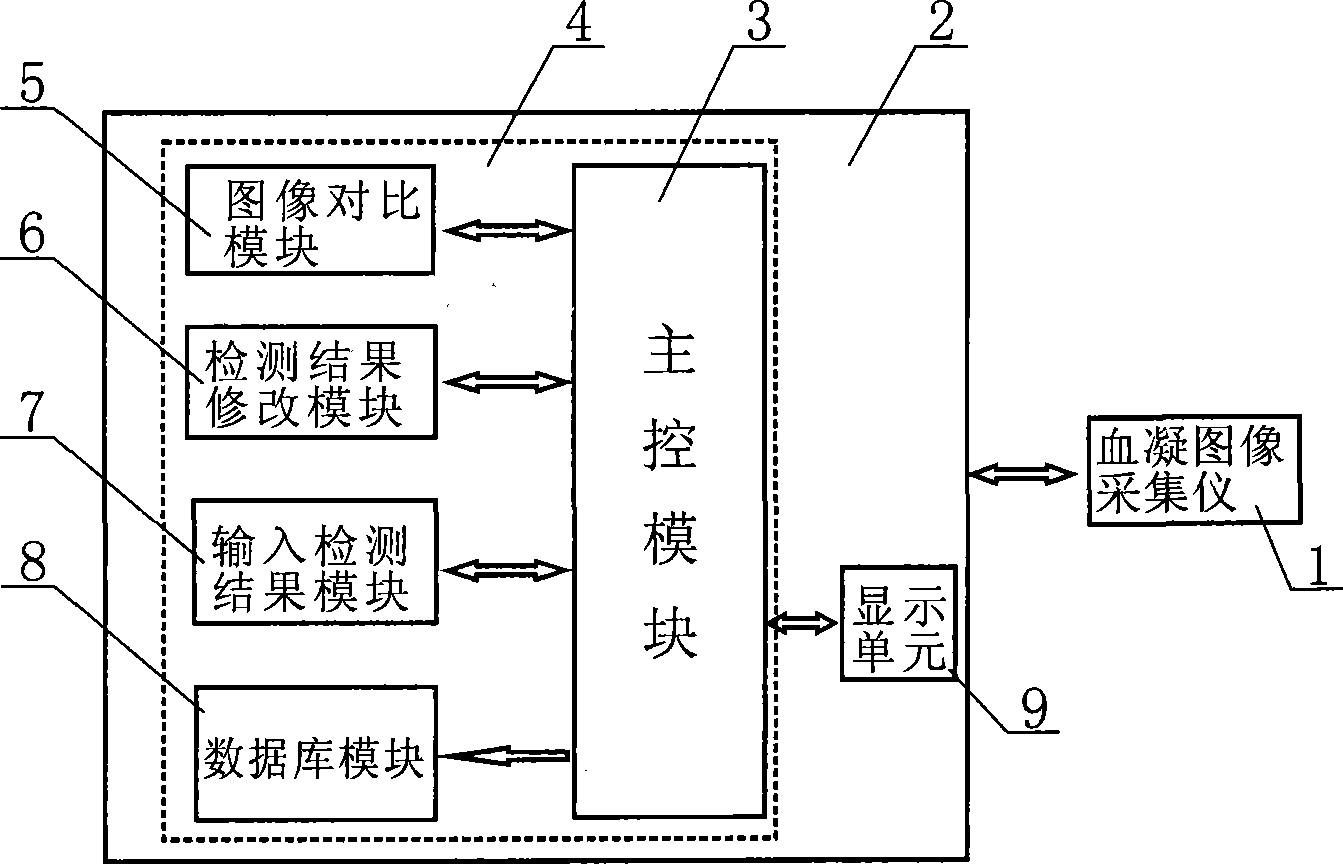
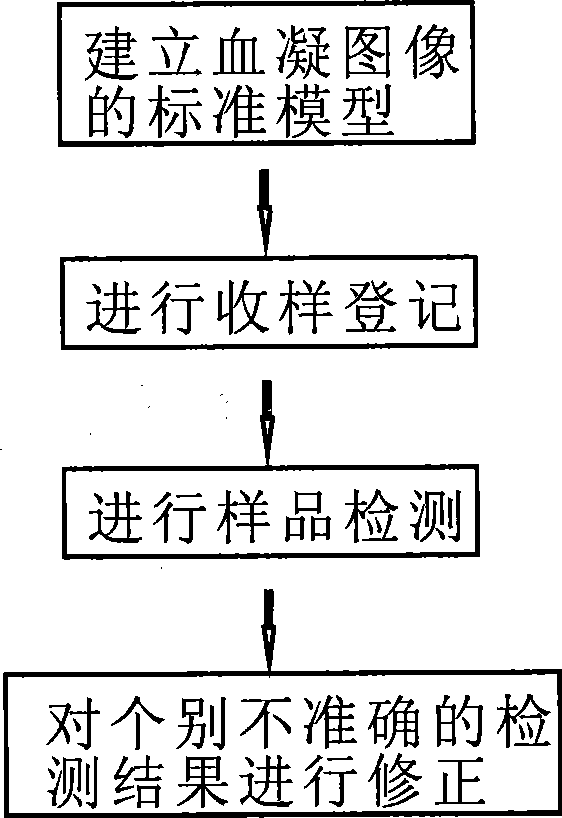
The invention discloses an analysis system for blood clotting in plague used for carrying out tests on immunological blood clotting or blood clotting inhibition to such plagues as bird flu, swine fever and foot and mouth disease. The invention comprises hardware and test program software, wherein the hardware comprises a blood clotting image acquisition instrument and a computer, and the software comprises a database module, an image comparison module and a master control module. The image comparison module compares an original image of a tested sample in the blood clotting test acquired by the blood clotting image acquisition instrument with a standard blood clotting image model in the database module; if the tested sample image acquired has the same characteristics in blood clotting with a certain standard blood clotting image, then the judgment result information corresponding to the standard blood clotting image is taken as the test result of the tested sample; and the test program software tests and analyzes the tested sample image and manages the test results data, thereby simplifying and facilitating the operation, raising standardization degree of the test, test precision and testing efficiency and being convenient for searching, filing, statistics and plague tracing.
Owner:杨傲冰
Polymerase chain reaction (PCR) primer pair for identifying H9 subtype avian influenza virus and application thereof
InactiveCN103667519AStrong specificityRapid detection meansMicrobiological testing/measurementDNA/RNA fragmentationLaryngotracheitis virusEpidemiologic survey
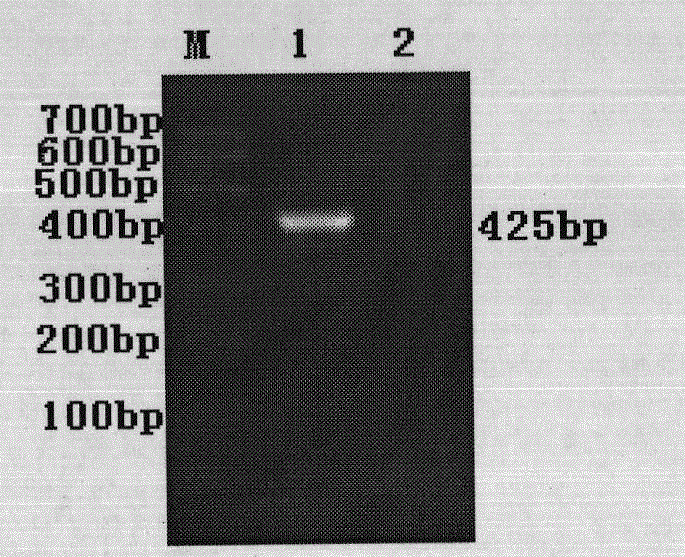
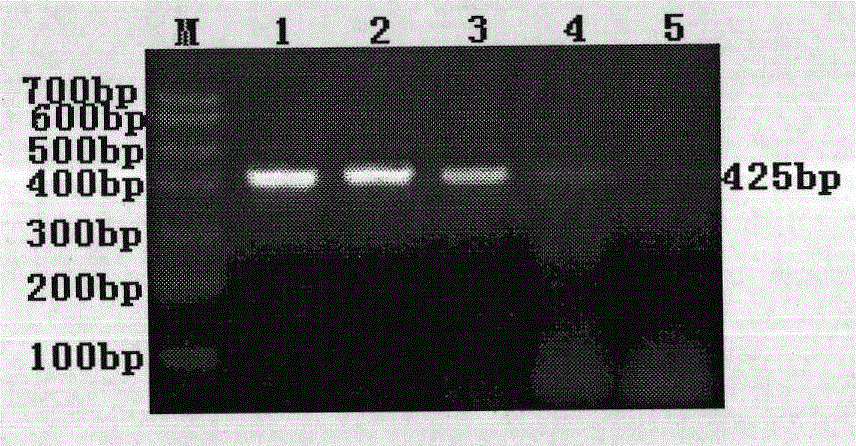
The invention discloses a polymerase chain reaction (PCR) primer pair for identifying an H9 subtype avian influenza virus (AIV) and application thereof. The PCR primer pair disclosed by the invention is composed of two single chain deoxyribonucleic acids (DNAs), wherein the two single chain DNAs are single chain DNAs shown in SEQ ID NO:1 and SEQ ID NO:2 in a sequence table. The HA gene of the H9 subtype AIV in a sample can be subjected to specific amplification, and the length of a target segment is 425bp. The method is free of cross reaction on H3, H4, H5 and other subtype AIVs, and a newcastle disease virus, avian infectious bronchitis, an infectious bursal disease virus, an infectious laryngotracheitis virus and the like; the minimum detectable quantity of virus allantoic fluid is 1*10<4.25>EID50 / 100mu L. Compared with the conventional methods such as a hemagglutination inhibition test of the virus and the like, the accordance rate of the identification result is 100%. A rapid, specific and sensitive detection means is provided for identification of the H9 subtype AIV. The PCR primer pair can be applied to rapid diagnosis of a disease caused by the H9 subtype AIV, and has a good application prospect in the aspects of clinical diagnosis and epidemiological investigation.
Owner:LIAOCHENG UNIV
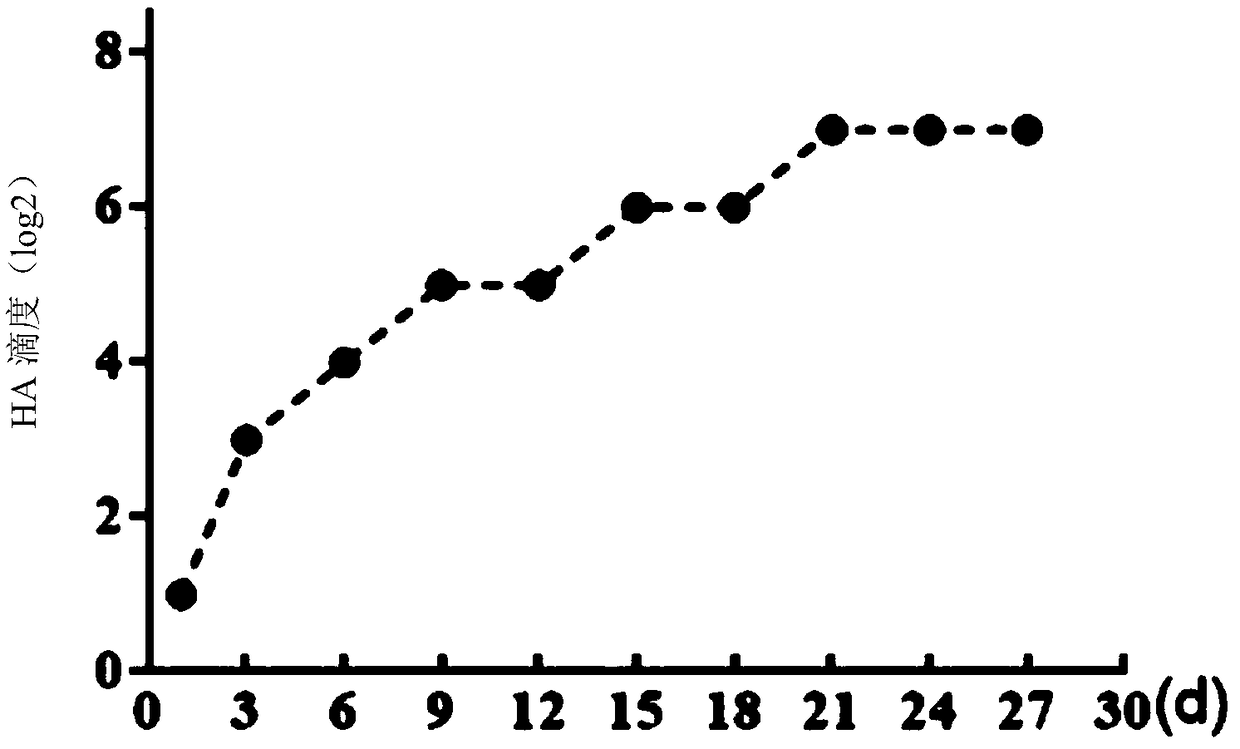
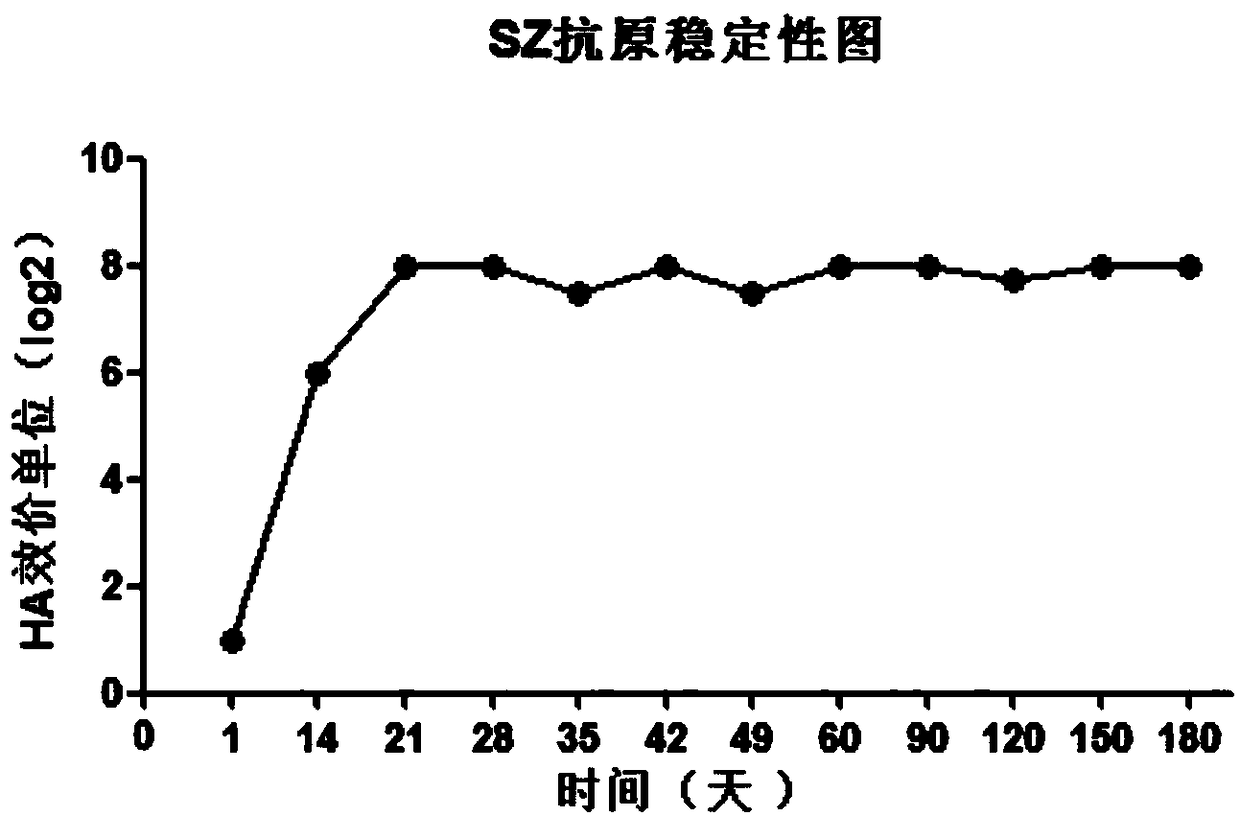
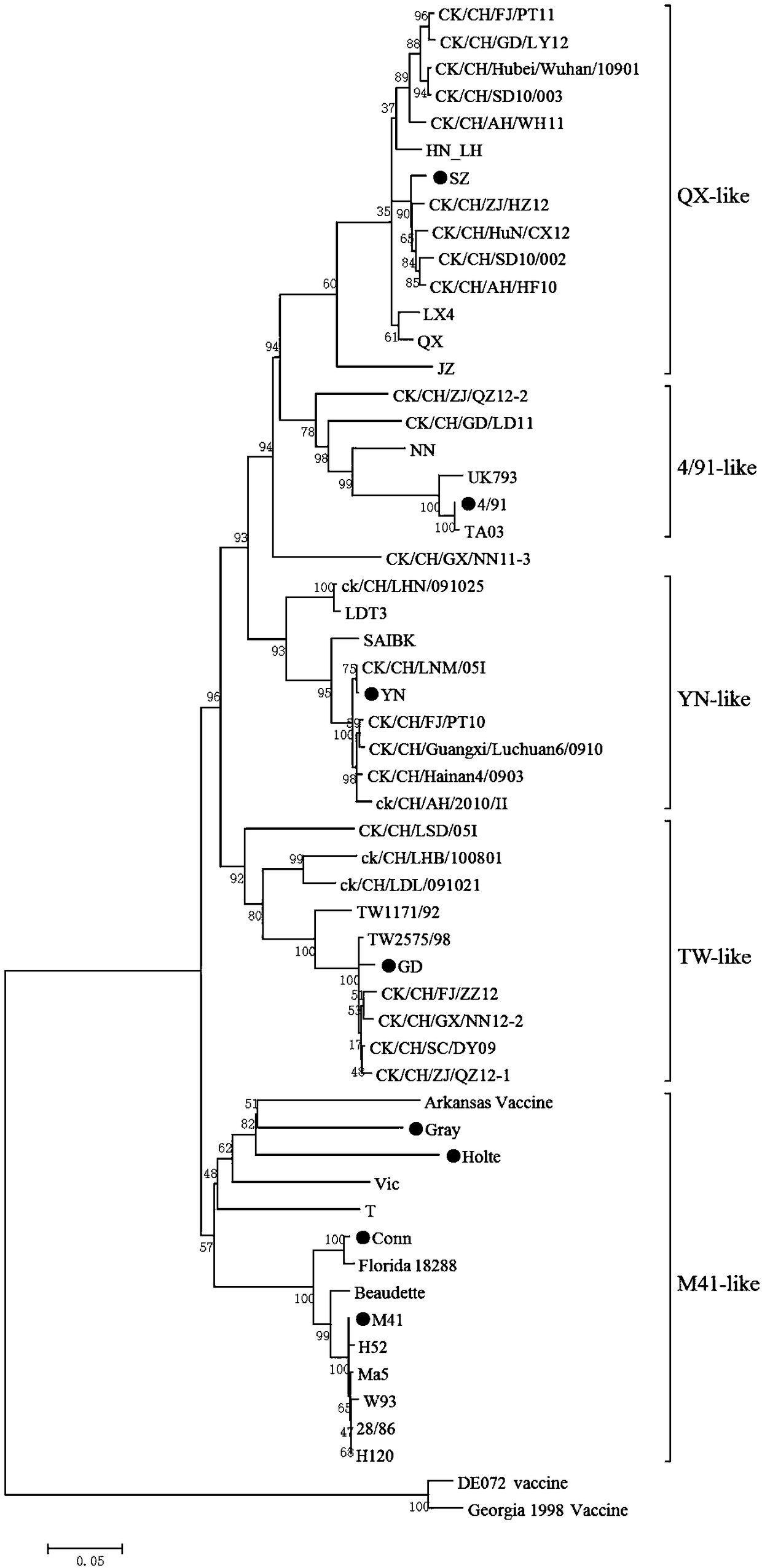
QX-type infectious bronchitis virus (IBV) hemagglutination inhibition test antigens, preparation method thereof and application
The invention relates to QX-type infectious bronchitis virus (IBV) hemagglutination inhibition test antigens, a preparation method thereof and application. A preparation method for an HI antigen comprises the following steps of inoculating 10<4>-10<7> EID<50> of QX-type IBV strains to chicken embryos, carrying out incubation for virus multiplication at a temperature of 37 degrees centigrade, after36-48h, taking out the chicken embryos, carrying out cooling for 6-16h at the temperature of 2-8 degrees centigrade, collecting chicken embryo allantoic liquid, uniformly mixing concentrated chickenembryo allantoic liquid with I-type phosphatase C, carrying out shaker action for 2-2.5h at the temperature of 37 degrees centigrade, and then carrying out placement for 21-35d at the temperature of 2-8 degrees centigrade to obtain antigens. The HI antigens and the detection method provided by the invention are characterized by high specificity, high stability, high sensitivity and high efficiency. Rapid detection can be carried out on clinical serum samples within 2h. The HI antigens and the detection method can be widely applied to quarantine and diagnosis of IB and detection of immunized antibodies. The HI method for detecting QX-type infectious bronchitis becomes feasible. A practical and scientific method for prevention of the IB is provided. The prevention and control over the disease in poultry production is guided well.
Owner:CHINA AGRI UNIV
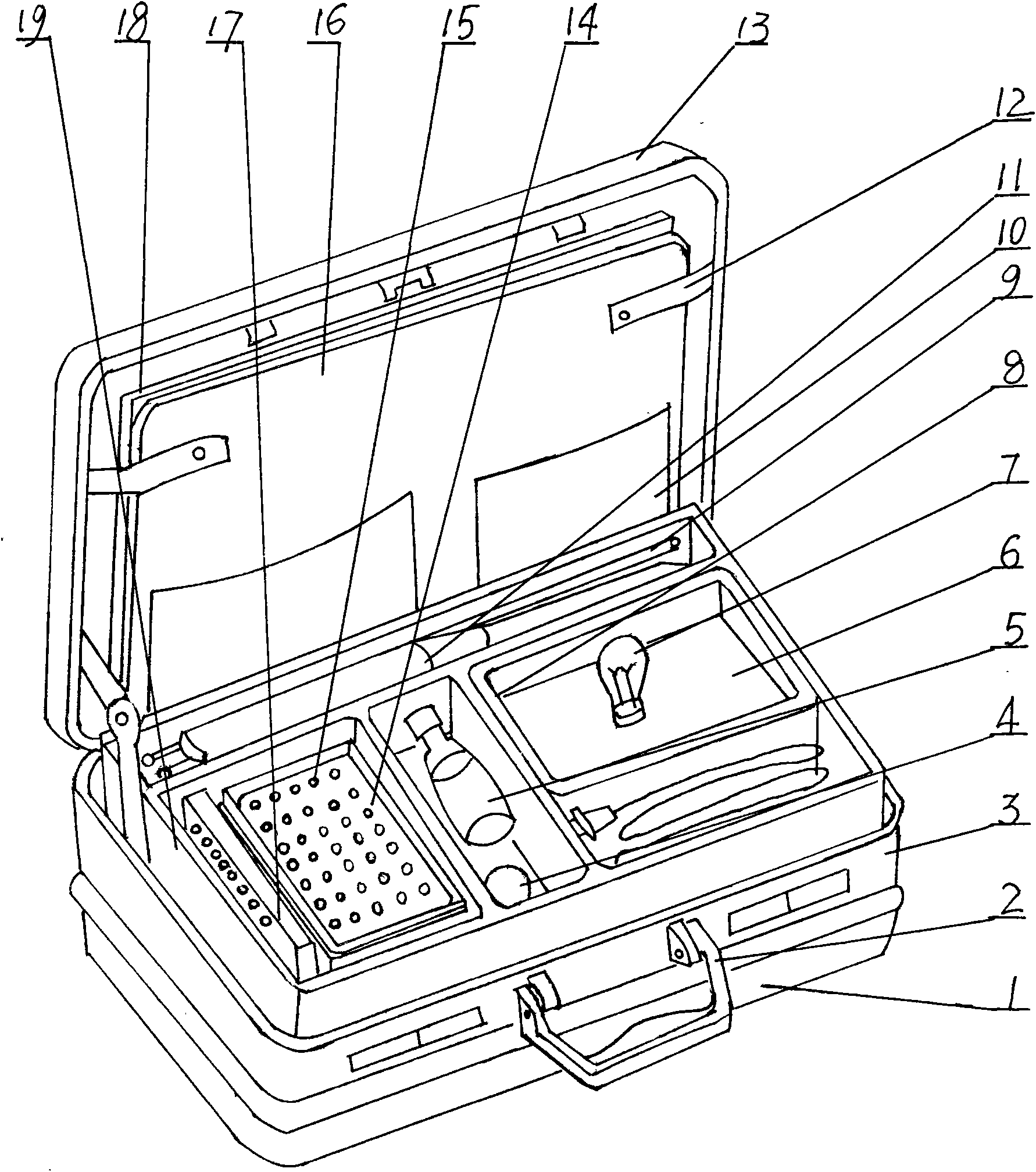
Method for producing working solution for virus hemagglutination inhibition tests and device applied thereby
The invention discloses a method for producing working solution for virus hemagglutination inhibition tests and a device applied thereby, and belongs to the veterinary speciality in the field of agriculture. The invention provides a method for producing working solution used on the scene for virus hemagglutination inhibition tests and a device applied thereby. The method comprises that: 100 to 300g of sodium citrate, 400g to 600g of glucose, 150 to 170g of sodium chloride, 1 to 5g of chromic chloride and 10 to 20g of sodium azide are added to 1,000ml of water, and pH is adjusted to 7.0 by NaOH. The device comprises a tank body consisting of a tank cover and a tank bottom with a handle, and has the structural key points that: plate glass is arranged in a baffle of the tank cover; a testing tool box is arranged on the tank bottom, and is provided with compartments inside; and the insides of the compartments are provided with medicament bottles, micro pipette tips, hemagglutination reactive plates, centrifugal tubes, a centrifuge tube shelf, suction tubes, plates and a pullorum disease detector with a bulb.
Owner:辽宁省动物疫病预防控制中心
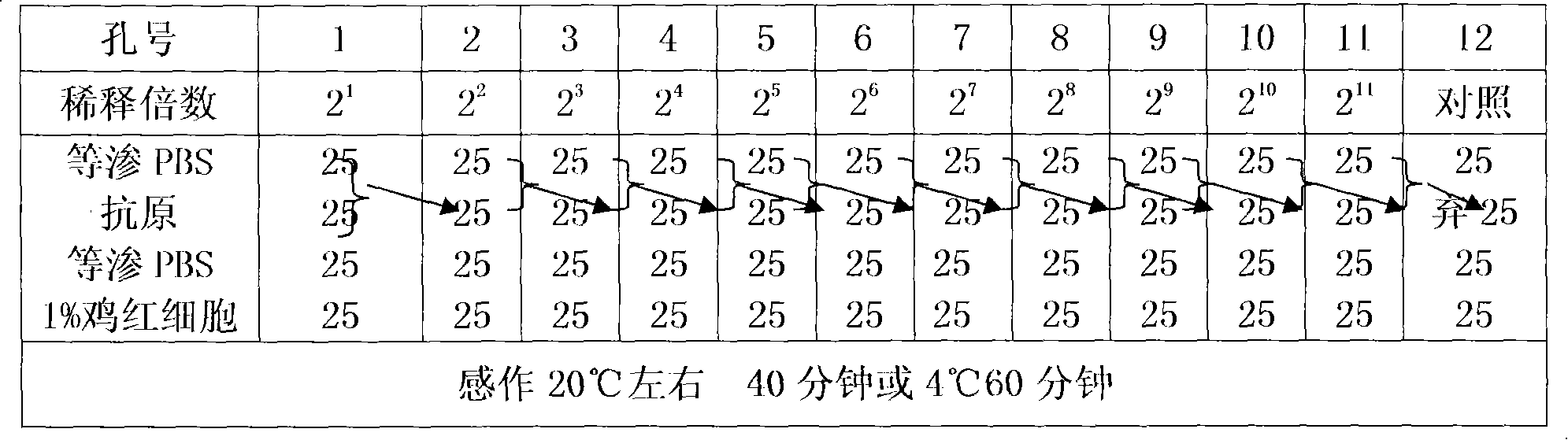
Detection method for avian influenza hemagglutination inhibition test
InactiveCN108088995AMonitor antibody levelsMonitor uniformityImmunoassaysSerum igeAvian influenza virus
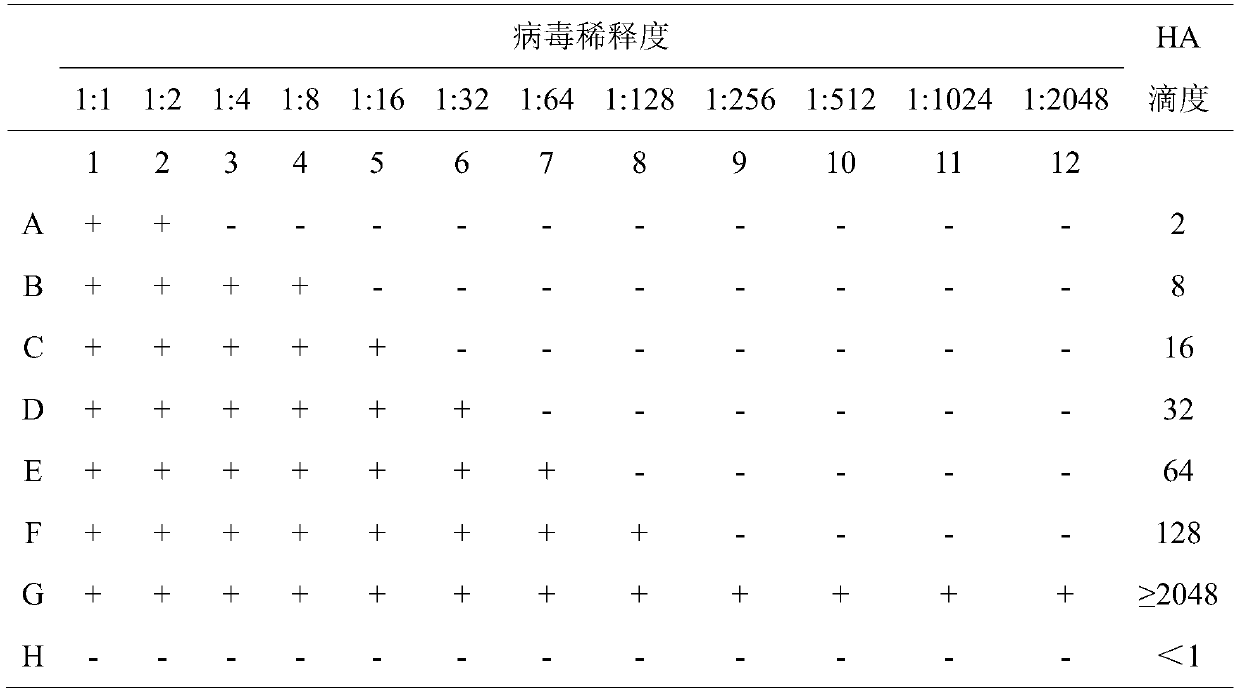
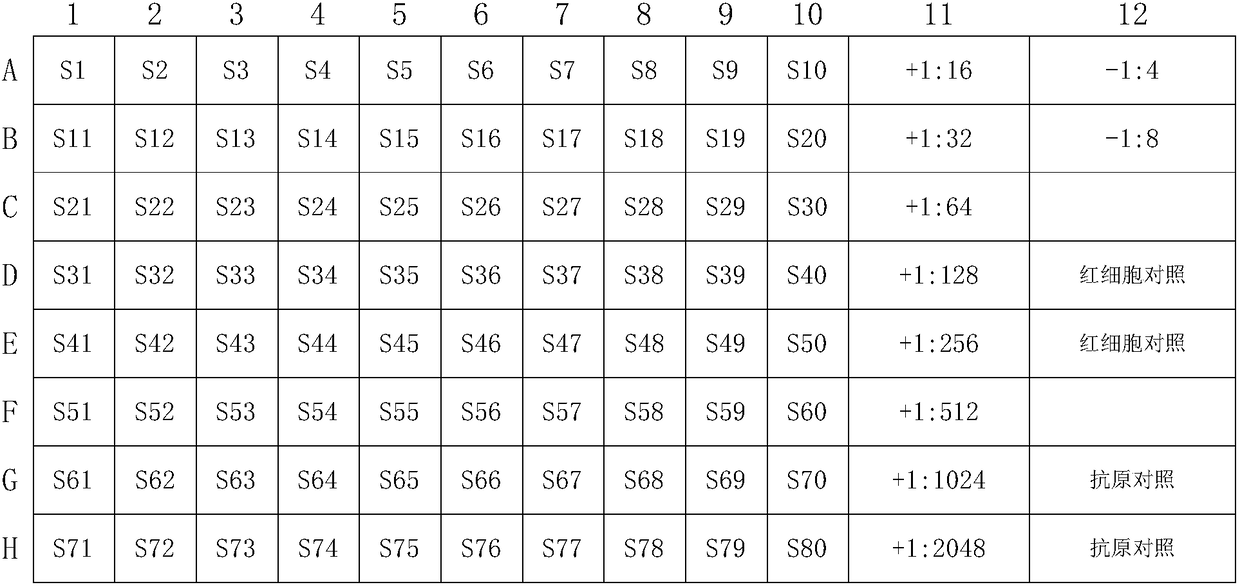
The invention discloses a detection method for an avian influenza hemagglutination inhibition test. The detection method comprises the following steps: S1, sequentially numbering a V-shaped micro-reaction plate from 1 to 12, sequentially adding 50 [mu]L of 4 units of an avian influenza virus antigen solution into each of the holes from 1 to 11; S2, respectively adding 50 [mu]L of detected serum into the first hole and the twelfth hole, mixing evenly, then absorbing 50 [mu]L to the second hole from the first hole, sequentially diluting to the tenth hole at multiple proportions, abandoning 50 [mu]L in the tenth hole; S3, sequentially adding 50 [mu]L of 1% chicken erythrocyte suspension into each hole from the twelfth hole to the first hole; S4, oscillating the V-shaped micro-reaction plate on a microoscillator, allowing standing still, incubating, and judging the agglutination condition of each hole when erythrocyte in the twelfth hole forms a button shape and sinks to the bottom of thehole. The detection method is efficient and fast, is high in accuracy, and is suitable for monitoring large chicken flocks by an enterprise.
Owner:广西凤翔集团股份有限公司
Hemagglutination inhibition test antigen for duck tembusu virus disease and preparation method thereof
The invention relates to a hemagglutination inhibition test antigen for a duck tembusu virus disease and a preparation method thereof. A duck hemorrhagic ovarian inflammation virus HB strain is preserved at the typical culture preservation centre in China with the preservation number being CCTCC V201122; the strain comprises a specific gene sequene, and the GenBank accession number of the strain is JF523187. The strain is inoculated againt a neonatal rat proliferating virus; the hemagglutination inhibition test antigen for the duck tembusu virus disease is prepared through the technologies of hemagglutination inhibition nonspecific material (lipoprotein) removal, inactivation, addition of a protective agent and the like. The invention further discloses the preparation method of the hemagglutination inhibition test antigen by a duck tembusu virus.
Owner:BEIJING ACADEMY OF AGRICULTURE & FORESTRY SCIENCES
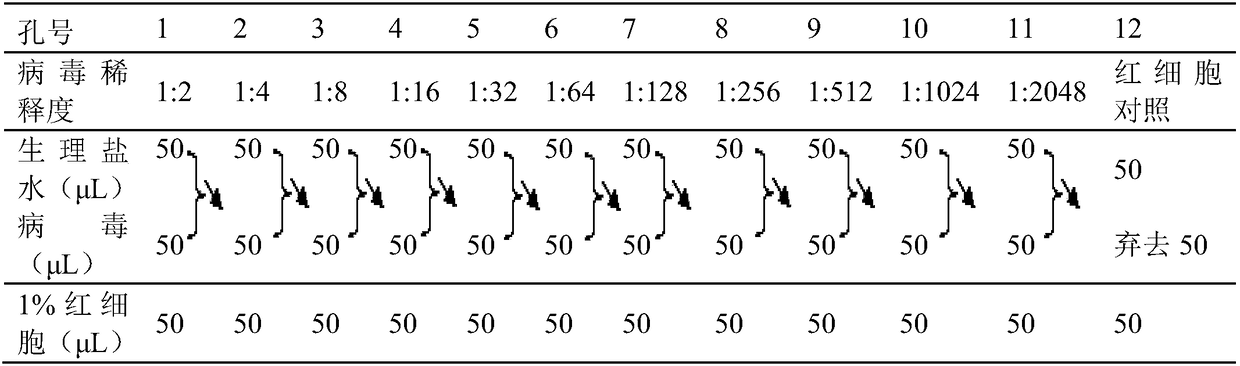
Influenza hemagglutination inhibition test detection method
PendingCN111323581ASolve the difficulty that the experiment cannot be carried outAvoid difficultiesBlood/immune system cellsBiological testingAntigenic analysisSpecific lectin
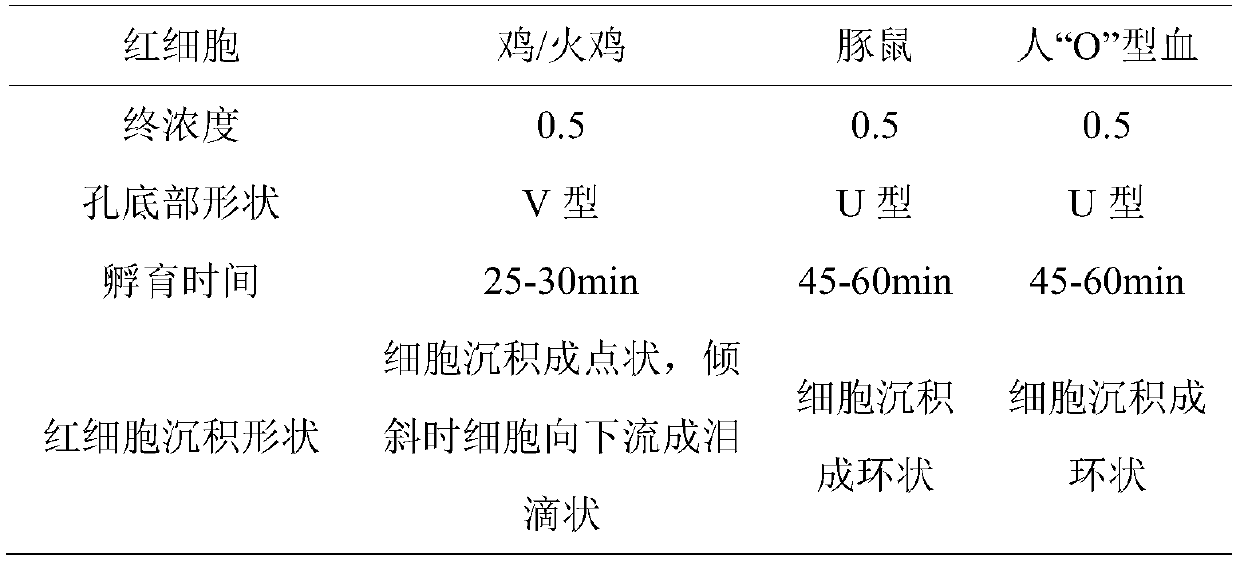
The invention belongs to the technical field of microbiological detection, and discloses an influenza hemagglutination inhibition test detection method which comprises the following steps: pretreatingstandard reference antiserum to remove non-specific inhibin and non-specific lectin in the serum; preparing an erythrocyte suspension; determining the hemagglutination titer of the influenza virus strain; preparing four hemagglutination unit antigens for an erythrocyte agglutination inhibition test; rechecking and titrating four hemagglutination unit antigens; and performing influenza virus identification and antigen analysis by using an HAI method to obtain the serum titer of the detected serum. According to the detection method, chicken erythrocyte, turkey erythrocyte and guinea pig erythrocyte are used as raw materials to prepare erythrocyte suspension, animal erythrocyte is used for replacing human erythrocyte, and the difficulty that an experiment cannot be conducted due to lack of erythrocyte is solved.
Owner:广州鸿泉生物科技有限公司
Hemagglutination and hemagglutination inhibition test reader
InactiveCN108663527AFast readAutomate managementMaterial analysis by optical meansBiological testingRed blood cellHemagglutination tests
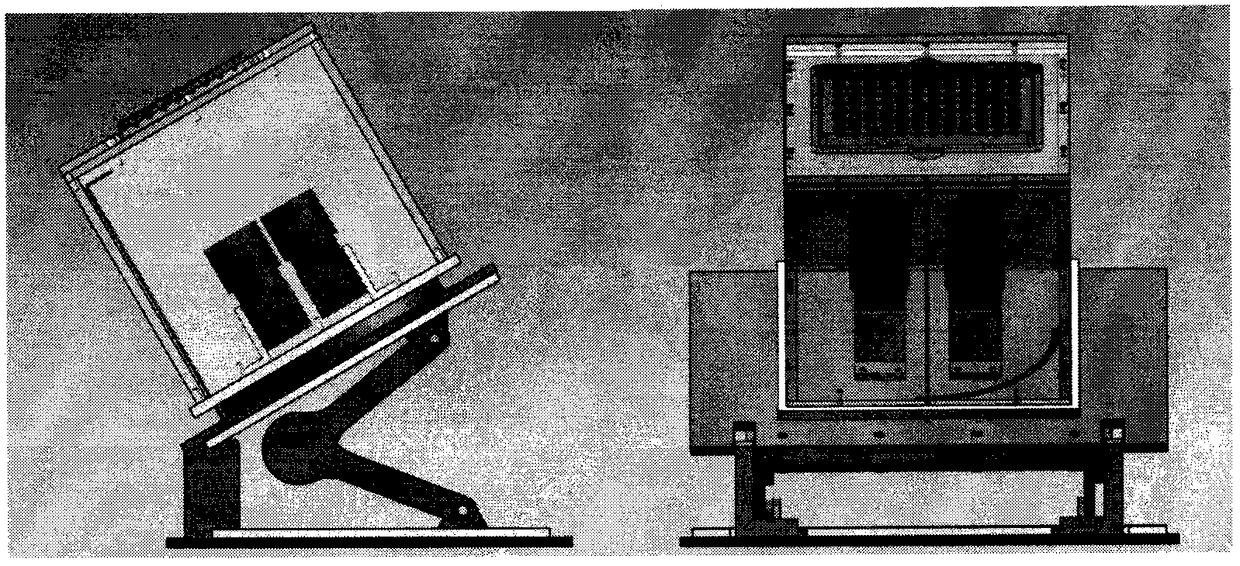
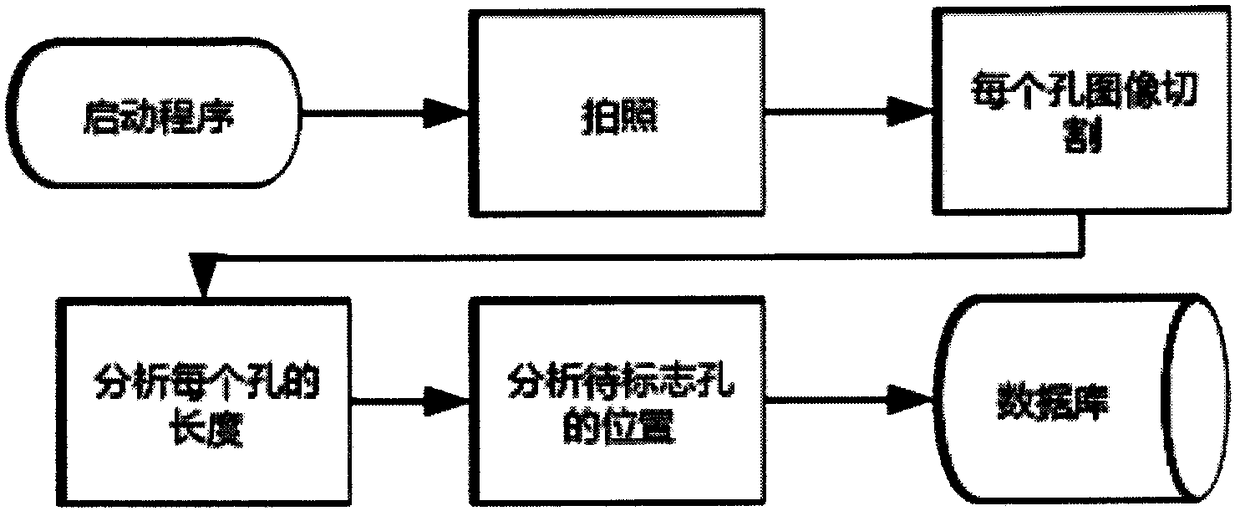
The invention provides an automatic interpretation tool for a hemagglutination and hemagglutination inhibition test result. The tool is a device for automatically generating the result. An interpretation method for the tool comprises the following steps: adjusting a hemagglutination plate support to make erythrocytes form a teardrop shape; carrying out zoning photographing on hemagglutination plate sample holes in a fixed object distance, calculating the teardrop length of the erythrocytes, and finding out a target hole; and processing obtained results, storing original images and interpretation results in a database, carrying out classified statistics according to the test results of a sample and test items, gathering the sample, and outputting the results by clicking a menu. The hemagglutination and hemagglutination inhibition test interpretation device and the statistical method thereof allow 96 samples to be simultaneously interpreted in 4 seconds, so the interpretation speed is much faster than that of manual operation, and the time and the labor are saved. Manual analysis and discrimination are not needed by using the tool, so visual fatigue and misjudgment are avoided, and individual differences are avoided; and the possibility of personnel in contact with pathogens is reduced, and the tool can be connected with a sample information management system to realize automaticmanagement.
Owner:南京市畜牧兽医站 +1
VIb subtype pigeon Newcastle disease positive serum standard substance and preparation method thereof
ActiveCN113376387AGuaranteed accuracyAccurate monitoringBiological material analysisBiological testingPositive controlEpidemiologic survey
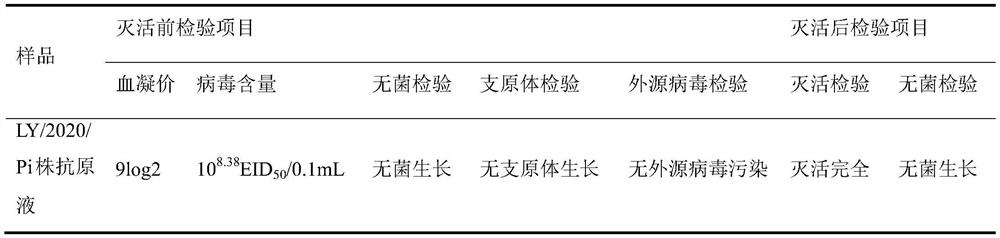
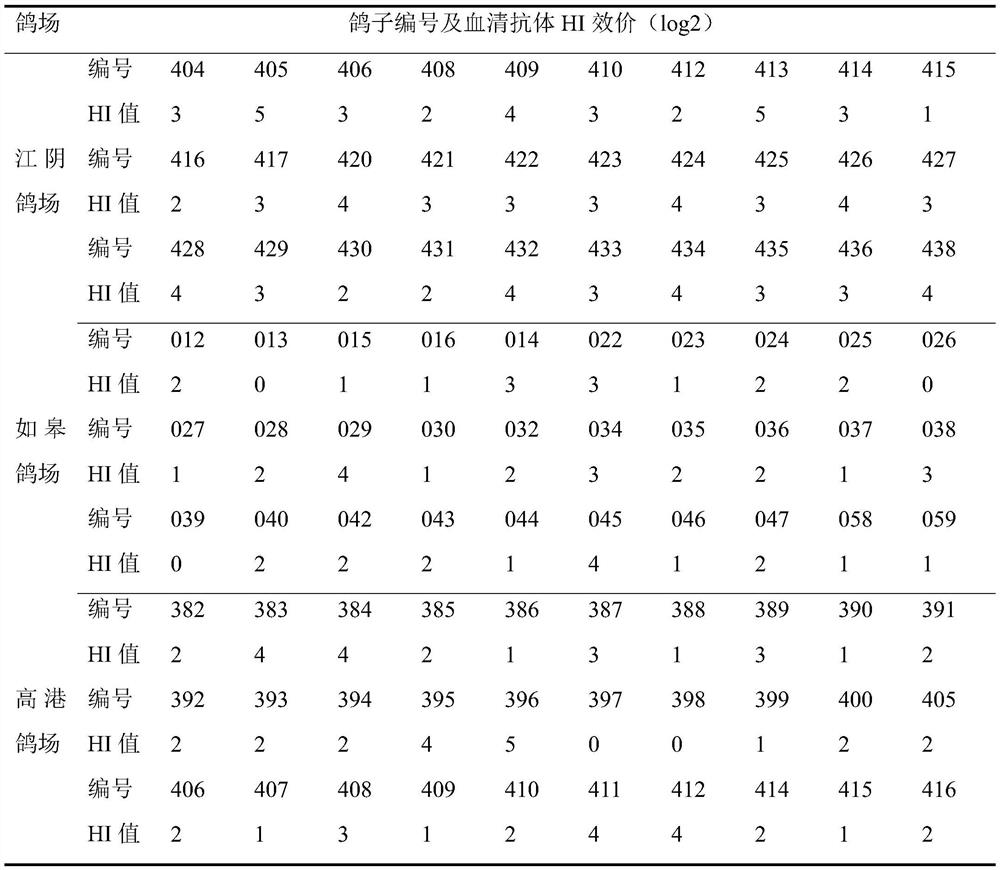
The invention relates to a preparation method of a VIb subtype pigeon Newcastle disease positive serum standard substance. The HI titer of the positive serum standard substance prepared by the method is not lower than 1:128, the neutralizing titer is not lower than 1:1600, the serum can completely neutralize the VIb subtype pigeon Newcastle disease virus, the positive serum standard substance is used for detecting the specificity of a positive control standard substance and a vaccine seed virus in a hemagglutination inhibition test and an exogenous virus, the accuracy of an antibody detection result can be ensured in the hemagglutination inhibition test, the antibody level of the immunized pigeons is fully reflected, and a guarantee is provided for accurate monitoring of VIb subtype Newcastle disease epidemic strains in pigeons, immunogenicity and specificity detection of pigeon Newcastle disease seed viruses, exogenous virus detection and vaccine immune efficacy evaluation. The invention fills the blank that no standard positive serum exists in the development of VIb subtype pigeon Newcastle disease vaccines and epidemiological investigation. The preparation method of the positive serum comprises the following steps: preparation of a pigeon Newcastle disease LY / 2020 / Pi strain inactivated vaccine, animal screening, animal immunization, and preparation, purification and inspection of the positive serum.
Owner:ZHONGCHONG XINNUO BIOTECH TAIZHOU CO LTD
VP2 gene and NP gene recombinant adenovirus and application thereof
ActiveCN108060141AVirus peptidesMicroorganism based processesDisk diffusion susceptibility testAgar diffusion test
The invention provides a VP2 gene and NP gene recombinant adenovirus and application thereof. The preparation method comprises the following steps: respectively amplifying VP2 genes and NP genes fromSD strains of IBDV (Infectious Bursal Disease Virus) and AIV (Avian Influenza Virus), cloning the obtained target genes into shuttle vectors pDC315-MCS-EGFP, performing co-transfection on a recombinant adenovirus shuttle plasmid pDC315-VP2-NP-EGFP, an adenovirus macroskeleton pBHGlox(delta)E1 and 3Cre in HEK293 cells by utilizing a lipofection transfection method, recombining and packaging, thereby obtaining the recombinant adenovirus pBH-VP2-NP-EGFP containing the VP2 genes and NP genes. The recombinant adenovirus is capable of stimulating the body to produce an antibody, the IBDV antibody content determined by an agar diffusion test reaches an effective antibody level, and the AIV antibody content determined by a hemagglutination inhibition test method is obviously superior to that in acontrol group pBH-EGFP; and the challenge test results show that the protection rate reaches 90%.
Owner:TIANJIN RINGPU BIO TECH
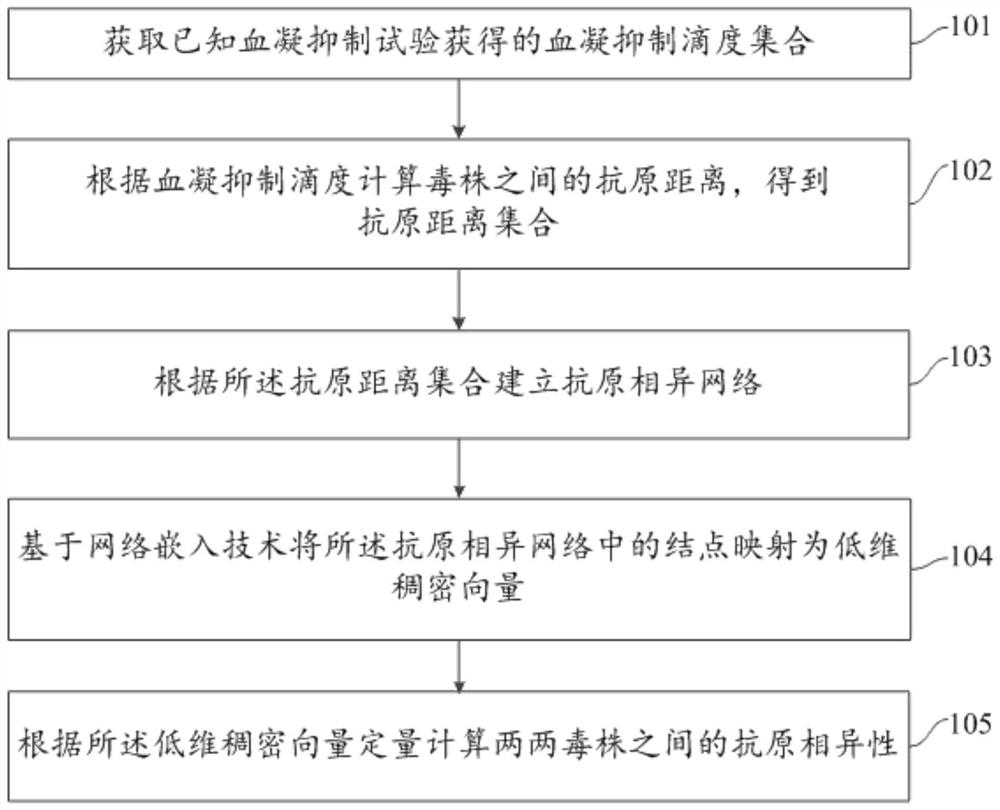
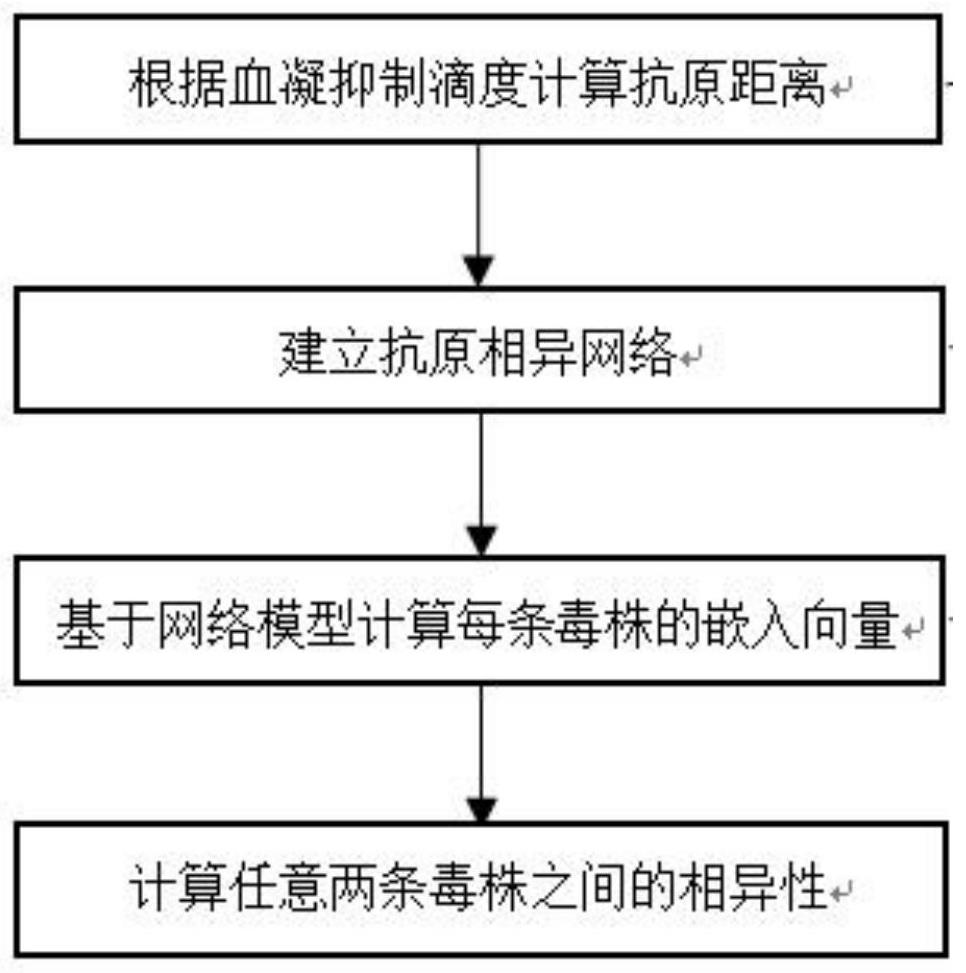
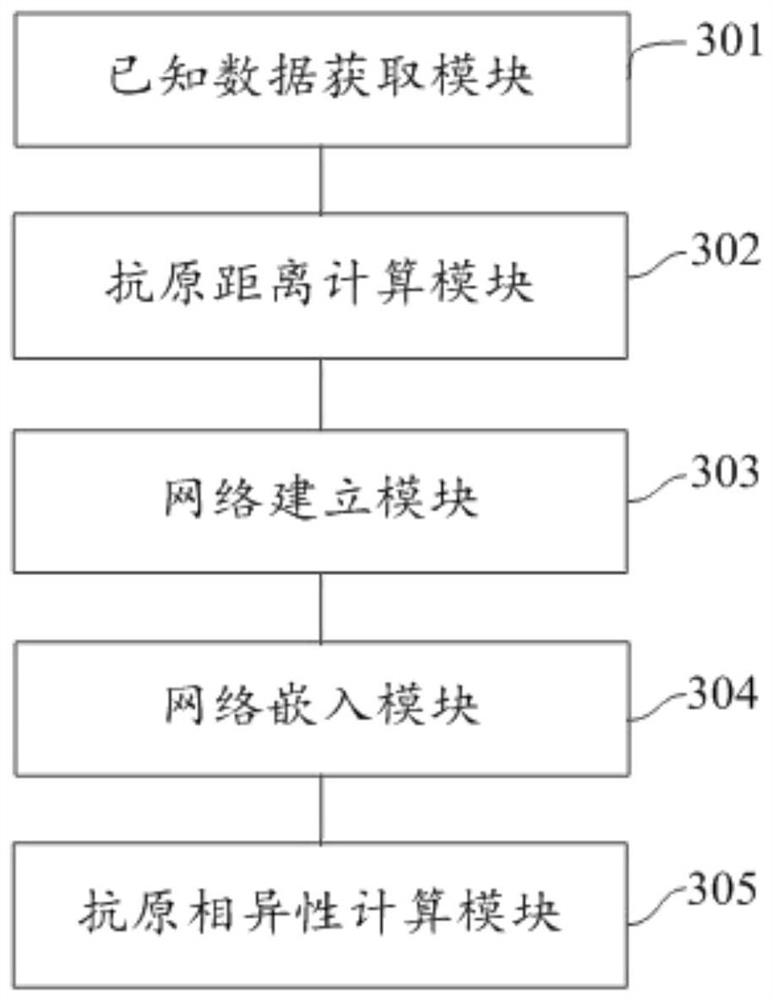
Influenza virus antigen dissimilarity calculation method and system
PendingCN114023443AImprove robustnessSupports antigenicity analysisMedical simulationEpidemiological alert systemsHemagglutinationHemagglutination Inhibition Tests
The invention relates to an influenza virus antigen dissimilarity calculation method and system. The method comprises the following steps: acquiring a hemagglutination inhibition titer set obtained by a known hemagglutination inhibition test, wherein the hemagglutination inhibition titer set comprises known hemagglutination inhibition titers; calculating the antigen distance between strains according to the hemagglutination inhibition titer to obtain an antigen distance set; establishing an antigen dissimilar network according to the antigen distance set; mapping nodes in the antigen dissimilar network into low-dimensional dense vectors based on a network embedding technology; and quantitatively calculating the antigen diversity between every two strains according to the low-dimensional dense vector. According to the method and the system, the antigen dissimilarity is modeled based on the network structure, the nonlinear feature learning capability of deep learning on the network structure is exerted, the antigen dissimilarity between every two strains can be quantitatively calculated, the robustness of antigen dissimilarity calculation is improved, and further, antigenicity analysis except for influenza antigen specificity can be supported, and good expandability and practicability are achieved.
Owner:YUNNAN UNIV
Mycoplasma gallisepticum antibody detection reagent, preparation method and application thereof
InactiveCN111707822AIn line with the domestic marketProductiveImmunoassaysMycoplasma antibodyMethyl violet
The invention relates to the technical field of poultry antibody detection reagents, particularly to a mycoplasma gallisepticum antibody detection reagent, a preparation method and application thereof. According to the invention, a mycoplasma gallisepticum culture solution is subjected to a series of treatment on, inactivating is performed, the inactivated antigen bacteria solution and a freeze-drying protective agent are uniformly mixed, and freeze drying is performed to obtain the mycoplasma gallisepticum antibody detection reagent; the reagent can be directly used for a hemagglutination inhibition test; a plate agglutination test can be carried out by adding any one coloring agent of methyl violet, amber red and crystal violet into the reagent containing 4-8HA unit of antigen; the hemagglutination value of the detection reagent reaches 26; and the detection reagent not only improves the hemagglutination titer of the mycoplasma gallisepticum antigen, but also has two purposes, is stable, sensitive, strong in specificity, long in storage life, simple to operate, convenient and fast, does not need special equipment and instruments, and can be used for efficacy test, epidemiologicalinvestigation, clinical sample detection and the like of mycoplasma gallisepticum vaccines.
Owner:兆丰华生物科技(南京)有限公司
A preparation method of hemagglutination inhibitory antigen of Avian bacillus paragallinarum and application of the antigen
ActiveCN109055253BExcellent hemagglutination activityHemagglutination activity hasBacteriaMicroorganism lysisHaemagglutination inhibitionHemagglutination tests
The invention provides a hemagglutination inhibition antigen of avibacterium paragallinarum, and a preparation method and application of the antigen. The antigen is prepared by adding staphylococcus hyicus filtrate into the avibacterium paragallinarum and treating. The hemagglutination inhibition antigen of the avibacterium paragallinarum can be applied to detection of an avibacterium paragallinarum antibody, and specifically can be used for preparing a reagent and a kit for a hemagglutination test or a hemagglutination inhibition test, and other immunological detection methods and reagents. The prepared hemagglutination inhibition antigen of the avibacterium paragallinarum is stable and has high hemagglutination titer; when the hemagglutination inhibition antigen is used for the hemagglutination test or the hemagglutination inhibition test, a result is more stable and easy to judge and the operability is strong.
Owner:BEIJING ACADEMY OF AGRICULTURE & FORESTRY SCIENCES
Antigen for hemagglutination inhibition test of duck Tembusu virus disease and preparation method thereof
The invention relates to a hemagglutination inhibition test antigen for a duck tembusu virus disease and a preparation method thereof. A duck hemorrhagic ovarian inflammation virus HB strain is preserved at the typical culture preservation centre in China with the preservation number being CCTCC V201122; the strain comprises a specific gene sequene, and the GenBank accession number of the strain is JF523187. The strain is inoculated againt a neonatal rat proliferating virus; the hemagglutination inhibition test antigen for the duck tembusu virus disease is prepared through the technologies of hemagglutination inhibition nonspecific material (lipoprotein) removal, inactivation, addition of a protective agent and the like. The invention further discloses the preparation method of the hemagglutination inhibition test antigen by a duck tembusu virus.
Owner:BEIJING ACADEMY OF AGRICULTURE & FORESTRY SCIENCES
Polymerase chain reaction (PCR) primer pair for identifying H9 subtype avian influenza virus and application thereof
InactiveCN103667519BStrong specificityRapid detection meansMicrobiological testing/measurementDNA/RNA fragmentationLaryngotracheitis virusSingle strand dna
Owner:LIAOCHENG UNIV
PCR (polymerase chain reaction) primer pair for identifying H3 subtype avian influenza virus and application thereof
InactiveCN102304591BStrong specificityEasy to operateMicrobiological testing/measurementMicroorganism based processesSingle strandEpidemiologic survey
The invention discloses a PCR (polymerase chain reaction) primer pair for identifying H3 subtype avian influenza virus and an application thereof. The PCR primer pair provided by the invention comprises two single-stranded DNAs (deoxyribonucleic acids), and the two single-stranded DNAs are the single-stranded DNA as shown in sequence 1 in a sequence table and the single-stranded DNA as shown in sequence 2 in the sequence table. The primer pair provided by the invention has high specificity when used for detecting the H3 subtype avian influenza virus, the sensitivity can reach1EID50 / 100 mu l, and the coincidence rate is 100% in comparison with the identification results obtained by conventional experimental methods, such as virus isolation, hemagglutination inhibition test and the like; and the primer pair can be used for detecting not only buccal swabs and cloacal swabs of sick poultry but also allantoic fluid of the sick poultry, the operation is simple, the popularization is easy, the basic level operation and the application are convenient, and the primer pair can become a useful detection tool for the diagnosis and the epidemiological investigation of the H3 subtype avian influenza virus disease.
Owner:CHINA AGRI UNIV
Recombinant adenovirus with vp2 gene and np gene and its application
The invention provides a VP2 gene and NP gene recombinant adenovirus and its application. The VP2 gene and NP gene are respectively amplified from the SD strains of IBDV and AIV, and the obtained target gene is cloned into the shuttle vector pDC 315 In ‑MCS‑EGFP, the recombinant adenoviral shuttle plasmid pDC was transfected by lipofection 315 ‑VP2‑NP‑EGFP and adenovirus backbone pBHGlox(delta)E1,3Cre were co-transfected into HEK293 cells, recombined and packaged to obtain recombinant adenovirus pBH‑VP2‑NP‑EGFP containing VP2 gene and NP gene. The recombinant adenovirus can stimulate the body to produce antibodies, and the IBDV antibody content measured by the agar expansion method reached an effective antibody level, and the AIV antibody content measured by the hemagglutination inhibition test method was significantly better than that of the control group pBH‑EGFP; the results of the challenge test showed that the protection rate up to 90%.
Owner:TIANJIN RINGPU BIO TECH
A screening method for hemagglutination and hemagglutination inhibition test
ActiveCN107328944BReduce demandImprove detection efficiencyBiological testingScreening methodQuarantine
The invention discloses a screening method for HA (hemagglutination) and HI (hemagglutination inhibition) tests. The screening method comprises steps as follows: dilution of antigen and serum; an HA test; screening of HI tests, wherein in screening of the HI tests, 80 parts of serum samples are detected once through one dilution plate and three reaction plates, positive samples are screened out, and the level of an immune antibody is finally judged. Compared with a conventional method, the method has the advantage that more than a half of antigens can be saved. More samples are detected by fewer antigens, so that the purpose of detecting the level of the antibody is achieved. The detection method is simple, the detection result is accurate and reliable, the detection efficiency is high, workload is greatly reduced, quantity demands for experimental persons are reduced, and the problems that a general laboratory is short of detection funds, detection reagents are limited and the detection number is large are well solved. The screening method is particularly applicable to primary organizations for epidemic prevention and quarantine of rural animals, the detection reagents are saved, and the detection speed is also increased.
Owner:王琴
Method for determining titer of swine flu inactivated vaccine
ActiveCN102735853BReduced measurement timeReduce operating errorsBiological testingRed blood cellPorcine influenza
The invention discloses a method for determining titer of a swine flu inactivated vaccine. The method comprises the following steps: immunizing a pig with a swine flue inactivated vaccine, collecting blood 21-28 days after immunization and separating blood serum; removing non-specific components in the serum to obtain a serum to be tested; diluting the swine flue inactivated antigen, and preparing a unit swine flue antigen diluent with a concentration of 4HA; and diluting the serum to be tested by multiple proportions, successively adding the 4HA unit swine flue antigen diluent and 1% red cells to conduct hemagglutination inhibition test, so as to completely inhibit highest dilution of the 4HA unit swine flu antigen serum at HI titer. The method has advantages of greatly shortened measurement time, little operation error, strong controllability, and small inter-batch difference, and also reduces detection errors caused by different levels of experimental animals in an animal challenge protection experiment for testing the titer.
Owner:GUANGZHOU SOUTH CHINA BIOLOGICAL MEDICINE +1
Automatic Newcastle Disease Detector
InactiveCN105974145BSimple structureCompact structureMaterial analysisNewcastle disease virus NDVRed Cell
The invention discloses a full-automatic Newcastle disease detector and relates to the technical field of animal medical equipment. The full-automatic Newcastle disease detector comprises a red blood cell supply unit, a virus liquid supply unit, a reagent supply unit, a reaction plate production unit, a vision interpretation unit, an interpretation region motion unit, a suction head supply unit, a sample feeding region motion unit and a single-shaft double-sliding block motion unit, wherein all the units are mounted on a base. According to the full-automatic Newcastle disease detector, Newcastle disease blood coagulation test and Newcastle disease blood coagulation inhibition test can be simultaneously carried out on Newcastle disease virus samples. The full-automatic Newcastle disease detector is simple and compact in structure and is suitable for batch, rapid and effective tests.
Owner:SHANDONG DOLANG TECH EQUIP
Full-automatic hemagglutination and hemagglutination inhibition test workstation
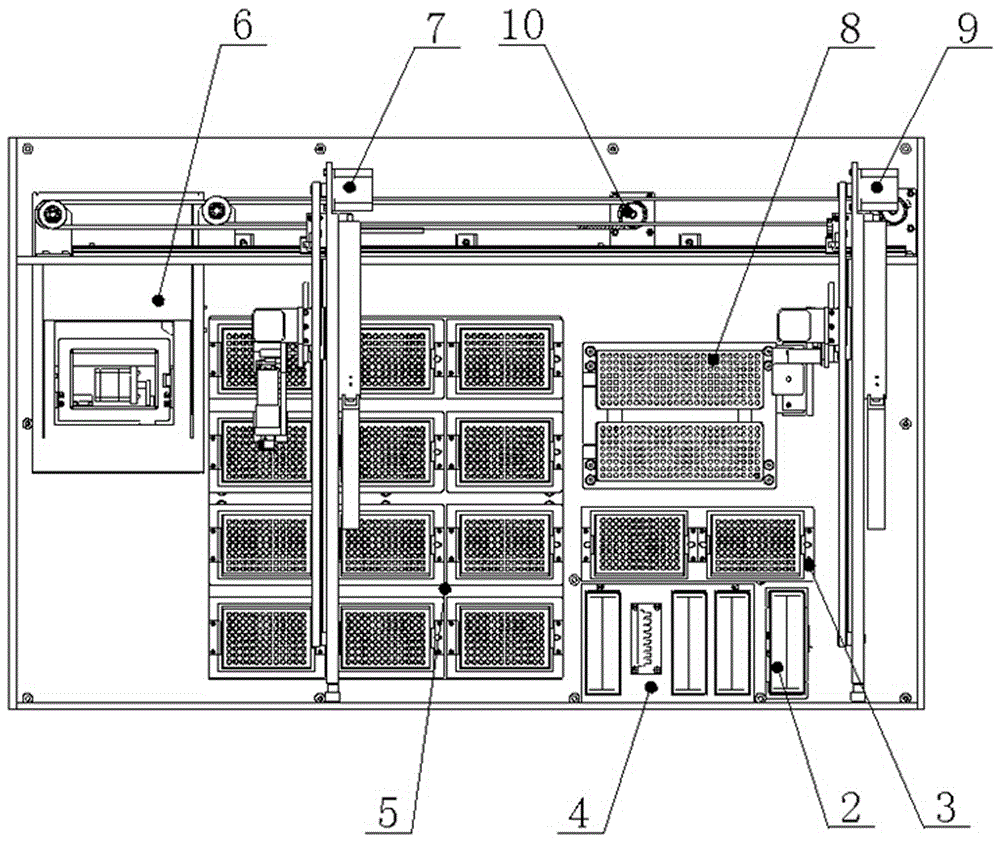
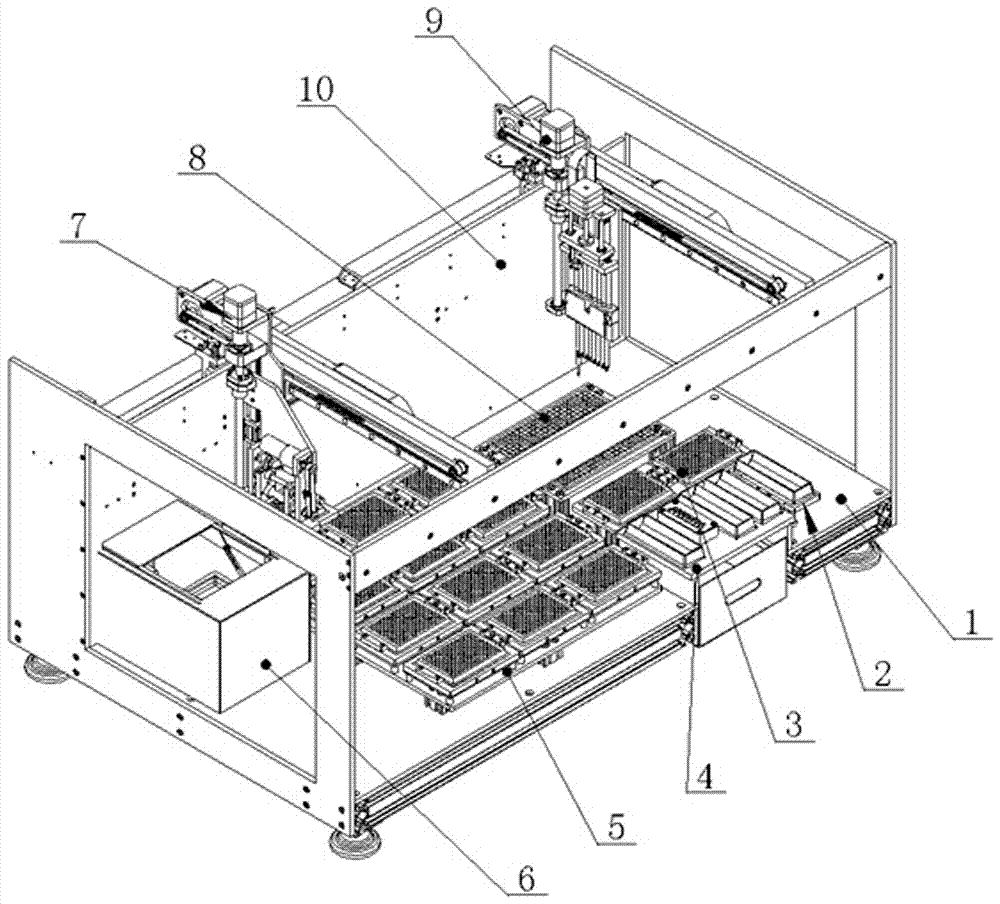
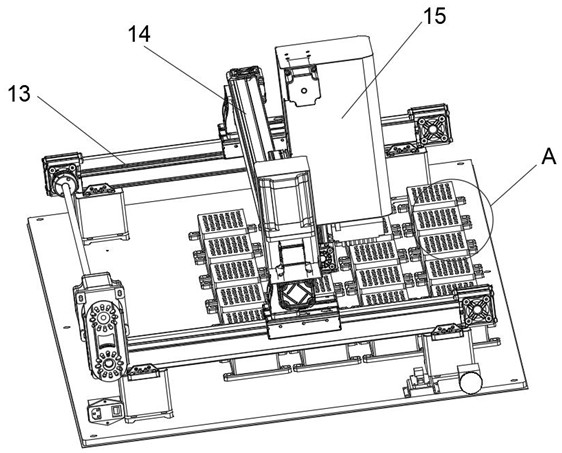
PendingCN114675044ARealize multi-position adjustment mobile processingImprove efficiencyMaterial analysisEngineeringStructural engineering
The invention discloses a full-automatic hemagglutination and hemagglutination inhibition test workstation which comprises a storage bottom box, the top of the storage bottom box is fixedly connected with a bottom plate, the top of the storage bottom box is fixedly connected with an upper shell, one side of the upper shell is connected with a door cover through a hinge, one side of the upper shell is provided with a cover opening mechanism, and the cover opening mechanism is connected with the door cover. The hemagglutination inhibition experiment sample detection device has the beneficial effects that the U-shaped fixing plates are additionally arranged, the inner sides of the two U-shaped fixing plates are mounted through different mounting blocks, the bottom plate can accommodate 24 plate positions, the efficiency of detecting a hemagglutination inhibition experiment sample is improved, and the detection accuracy is improved. The time and the cost of manual operation are greatly reduced; by adding the X-axis linear module, the Y-axis linear module and the Z-axis linear module, multi-position adjusting and moving treatment of the fixed box and the pipetting cylinder is realized, and the pipetting efficiency is improved.
Owner:南京应节生物科技有限公司
A rapid antigen detection method for inactivated oil emulsion vaccine against avian influenza finish products
ActiveCN103235139BImprove accuracyHigh-precision detectionBiological testingOil emulsionVaccine antigen
The present invention discloses a rapid antigen detection method for inactivated oil emulsion vaccine against avian influenza finish products, and the method comprises the following steps of: 1) mixing uniformly isopropyl myristate and the inactivated oil emulsion vaccine against avian influenza by thoroughly shaking, centrifuging, and separating a water phase layer; 2) performing HA titer detection to the water phase of the vaccine obtained in the step 1); 3) according to the detection result of the HA titer detection of the water phase of the vaccine in step 2), taking the water phase of the inactivated oil emulsion vaccine against avian influenza of step 1) to prepare a 4HAU vaccine antigen diluent; and 4) performing hemagglutination inhibition tests by using the 4HAU vaccine antigen diluent prepared in step 3), wherein a HI titer is expressed as the highest dilution serum that completely inhibits the 4HAU antigen. The method of the invention does not destroy the hemagglutination titer and antigenicity of vaccine antigens, can quickly and accurately determine the HI titer of the water phase of the inactivated oil emulsion vaccine against avian influenza finish products and analyze differences in antigenicity, and reagents in use are safety and non-toxic for human and environment, and are cheap and readily available.
Owner:ZHAOQING DAHUANONG BIOLOGIC PHARMA +2
Positive serum national standard substance used for newcastle disease hemagglutination inhibition test and preparation method thereof
The invention relates to a positive serum national standard substance used for a newcastle disease hemagglutination inhibition test and a preparation method thereof. In the positive serum national standard substance used for the newcastle disease hemagglutination inhibition test, Newcastle Disease Virus (NDV) La Sota strain virus and inactivated vaccine immune SPF (Specific Pathoge Free) chicken are utilized to prepare hyperimmune serum to be used as a candidate, through the processes of candidate detection, standard substance preparation, detection, split charging, freeze-drying and the like, the positive serum international standard substance used for the newcastle disease hemagglutination inhibition test obtained by NIBSC (National Institute for Biological Standards and Control) is taken as a reference to carry out a red blood cell agglutination inhibition test to determinate a valence of the standard substance, and through a uniformity test, a stability test, cooperation calibration and data statistics, a constant valence value is ensured to be accurate, and the positive serum national standard substance used for the newcastle disease hemagglutination inhibition test is prepared. The method disclosed by the invention makes the standard substance preparation more scientific, rigorous and perfect according to the standard substance preparation process.
Owner:CHINA INST OF VETERINARY DRUG CONTROL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com