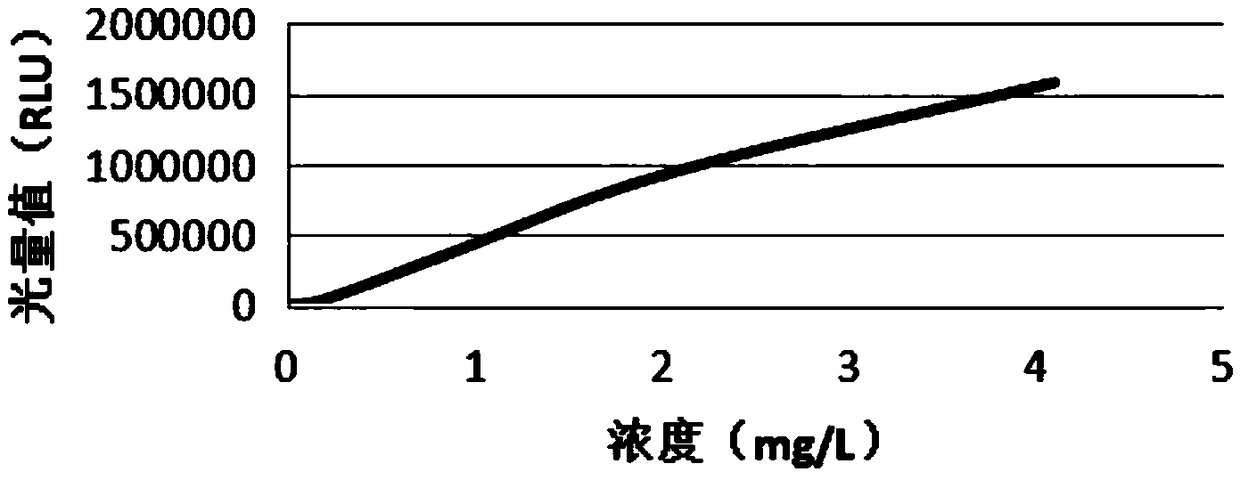
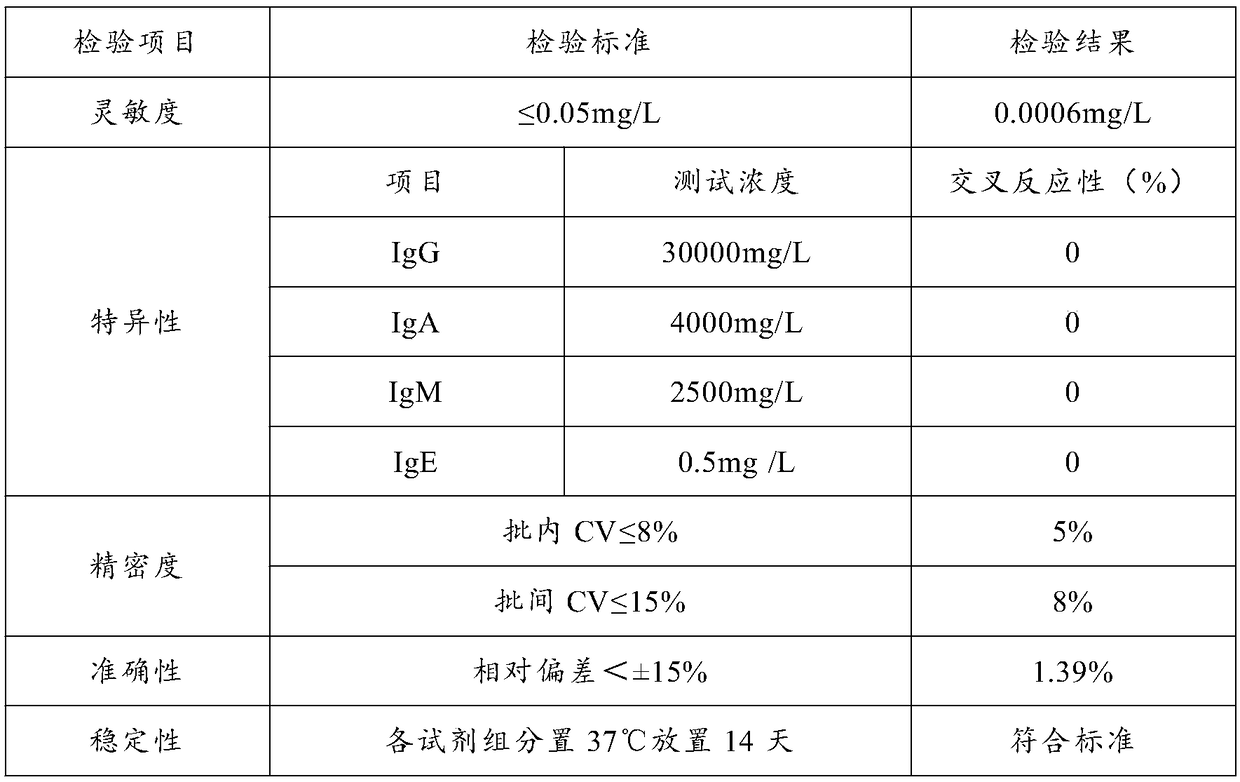
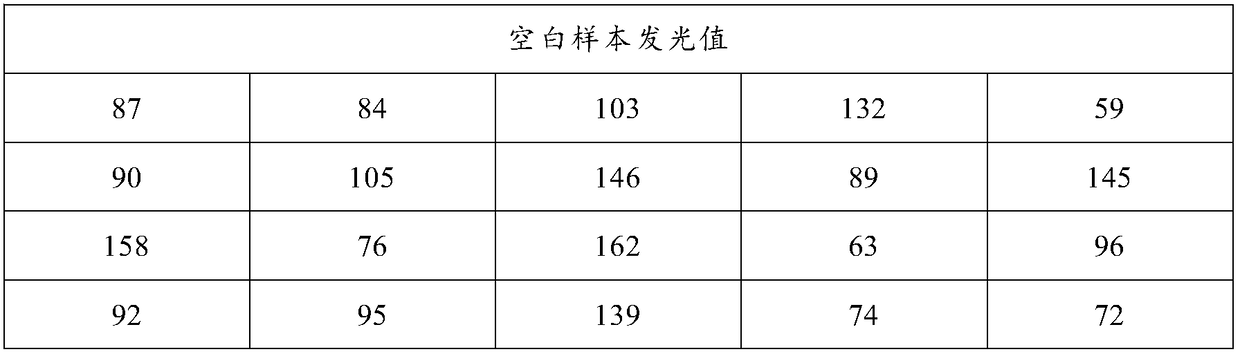
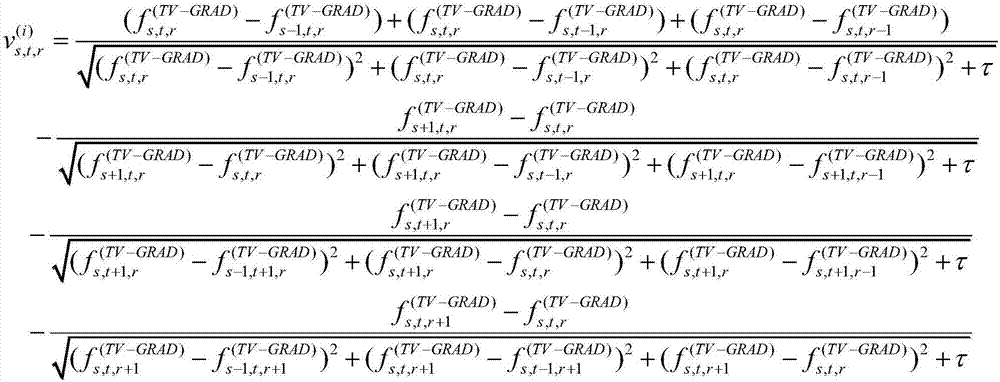Patents
Literature
48results about How to "Rapid detection means" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method for automatic identification and detection of defect in composite material
InactiveCN102692429ARealize automatic identification and detectionImprove performanceMaterial flaws investigationNeural learning methodsTime informationSingular value decomposition
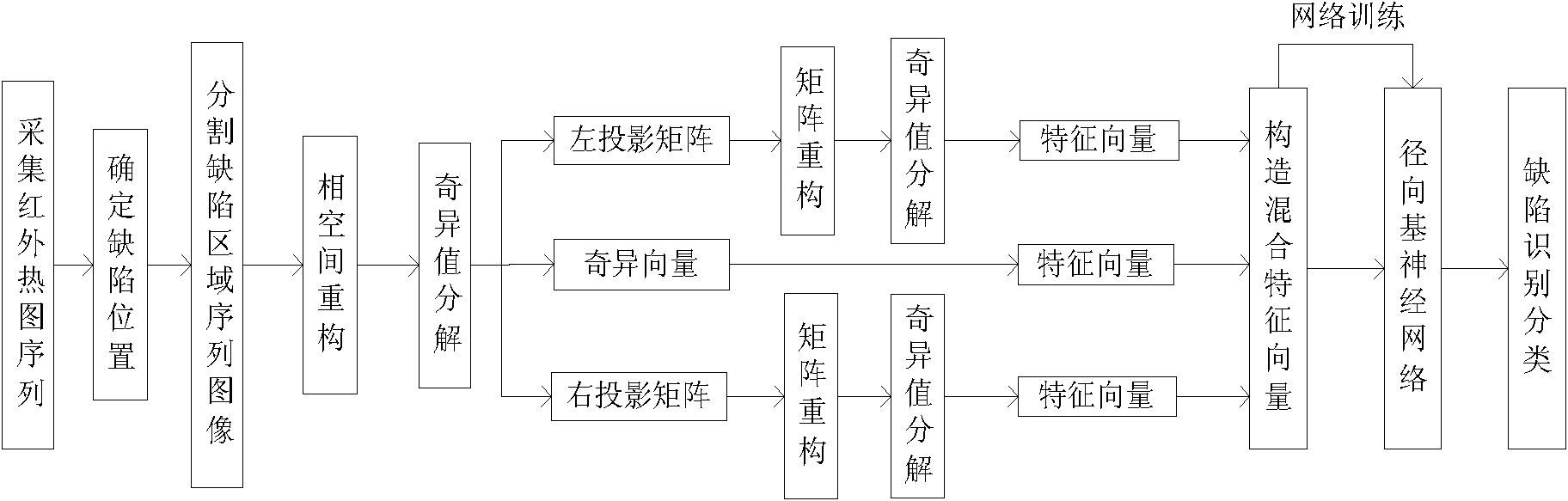
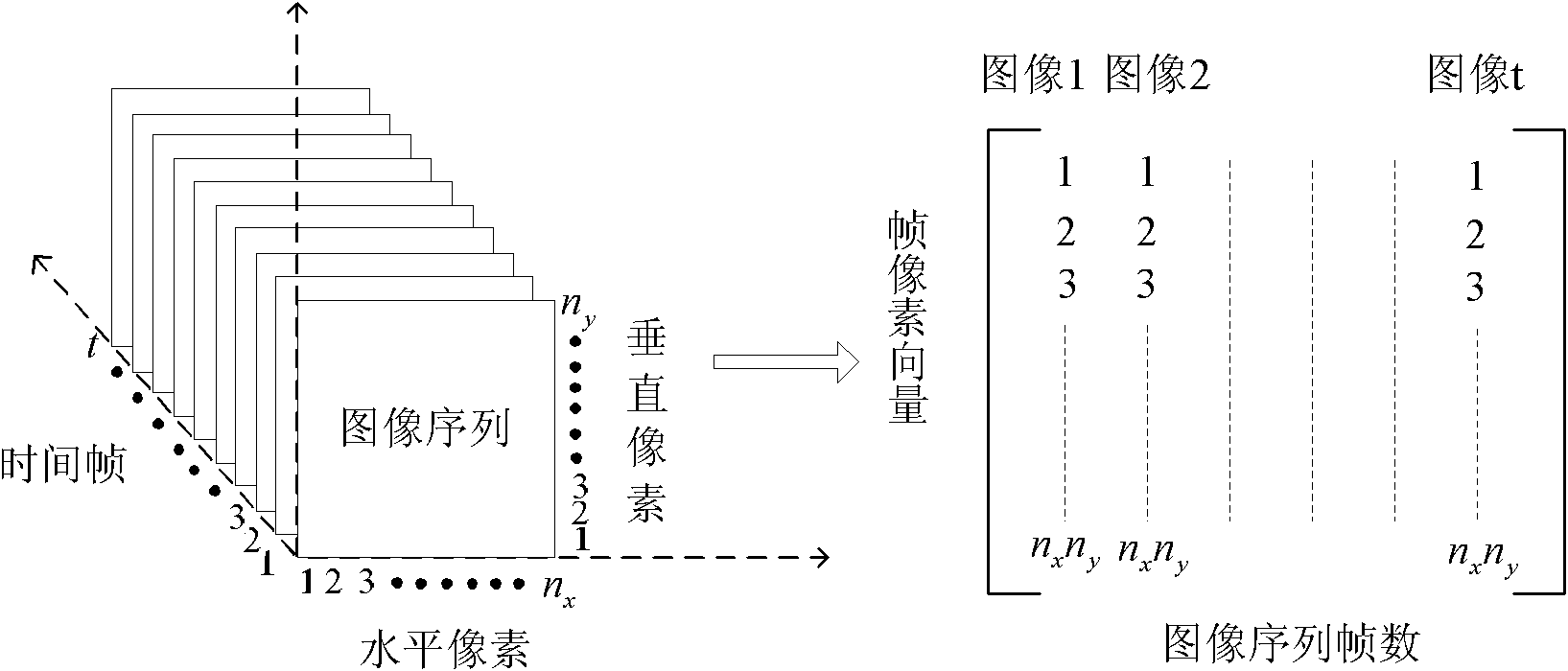
The invention relates to a method for automatic identification and detection of defects in a composite material. The method comprises steps of: detecting the composite material to generate an infrared image by using infrared thermal wave nondestructive testing equipment; conducting phase space reconstruction on the infrared sequence image to determine defect position of the composite material and segment defect area of the image; conducting phase space reconstruction on the infrared sequence image with defect area and carrying out singular value decomposition to obtain a singular matrix, and left and right projection matrixes; carrying out matrix reconstruction again on the two projection matrixes; extracting algebraic characteristics of time information and space information of the defect through singular value decomposition; constructing mixing characteristic vector as characteristic symptom of the defect; and utilizing results from a nerve network classifier to complete the identification and classification determination. The method of the invention can realize automatic identification and detection on defect in the composite material, carry out rapid detection on damage type of the composite material and provide rapid detection means according to usage condition of the composite material, and has critical reality meaning and research value.
Owner:SHENYANG INST OF AUTOMATION - CHINESE ACAD OF SCI
UPLC-MS/MS simultaneous flux detection method for multiclass veterinary drug residue in raw fresh milk
InactiveCN104764816ASensitive detection meansHigh sensitivityComponent separationQuantitative determinationFiltration
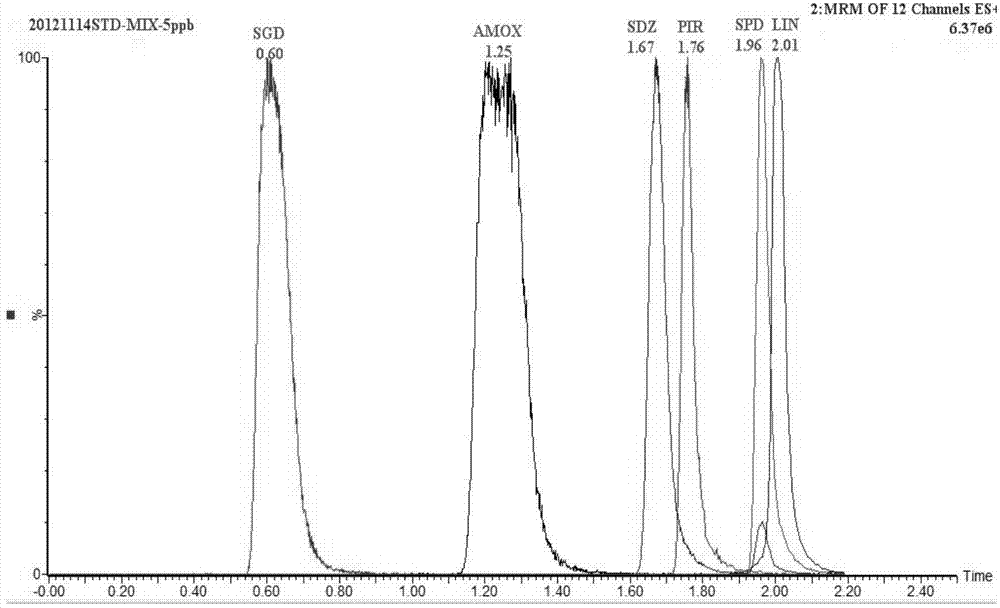
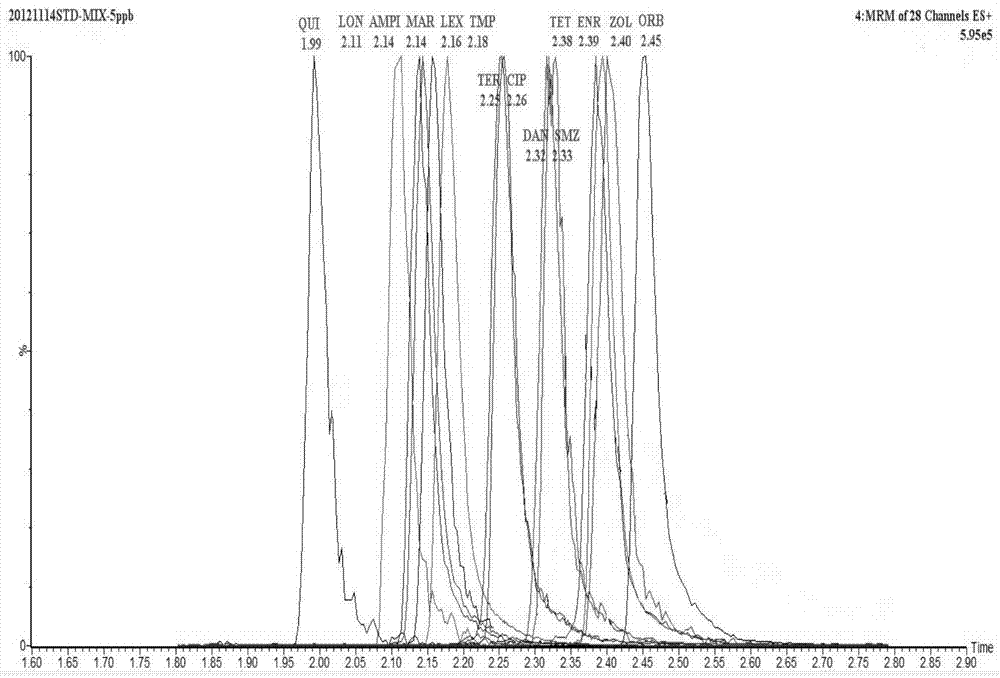
The invention discloses a UPLC-MS / MS simultaneous flux detection method for multiclass veterinary drug residue in raw fresh milk. The method includes the steps of: (1) extracting a veterinary drug compound in a to-be-detected sample to obtain an extracted solution; (2) concentrating the extracted solution, and then regulating the pH to 7.5-10.0 to obtain a column passing solution; (3) activating a solid phase extraction column; and (4) loading the column passing solution on the activated solid phase extraction column, leaching the solid phase extraction column with leacheate, then conducting elution with eluent, collecting the eluent, carrying out concentration, redissolving and filtration by a filter membrane, then performing qualitative and quantitative determination by UPLC-MS / MS. The method provided by the invention can simultaneously detect 38 veterinary drug residue in raw fresh milk, and has the characteristics of high sensitivity and low detection limit. The matrix standard curve correlation degree, the standard addition recovery rate of the method and the intra-day and inter-day precision are all accord with the China, European Union and international veterinary drug residue analysis method requirements. The method provided by the invention provides efficient and accurate detection means for raw fresh milk veterinary drug residue risk analysis and warning research.
Owner:INST OF ANIMAL SCI OF CHINESE ACAD OF AGRI SCI +1
Processing method and processing apparatus for sequencing data
ActiveCN105844116AEliminate dependenciesAvoid data fluctuationsSequence analysisSpecial data processing applicationsNucleotide sequencingBiology
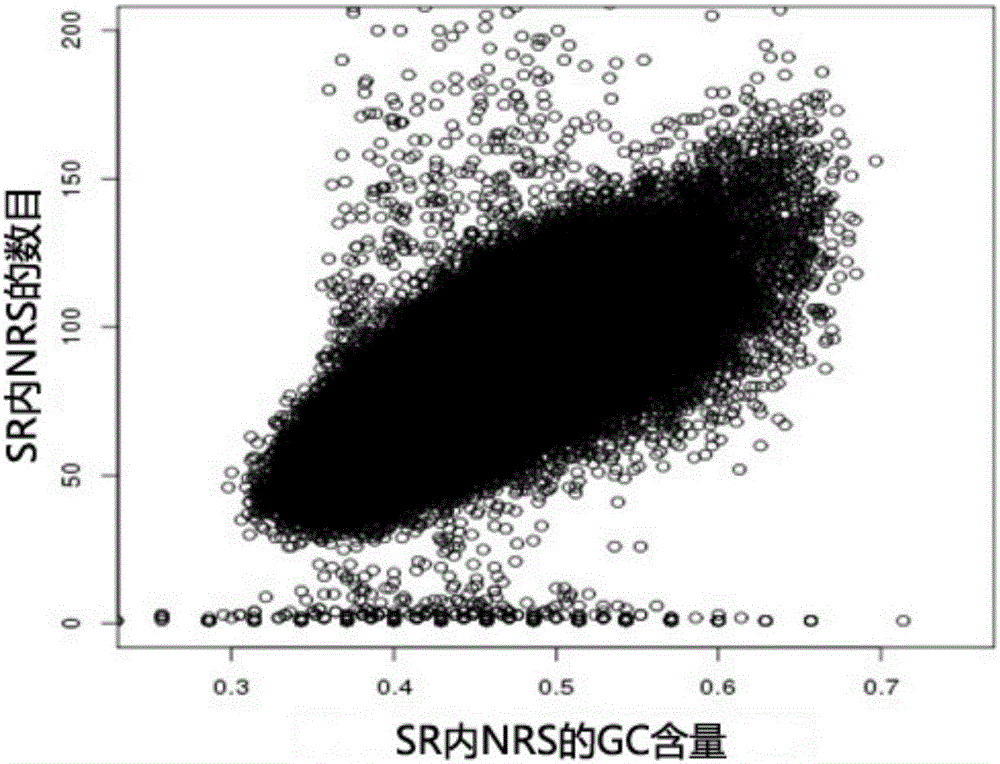
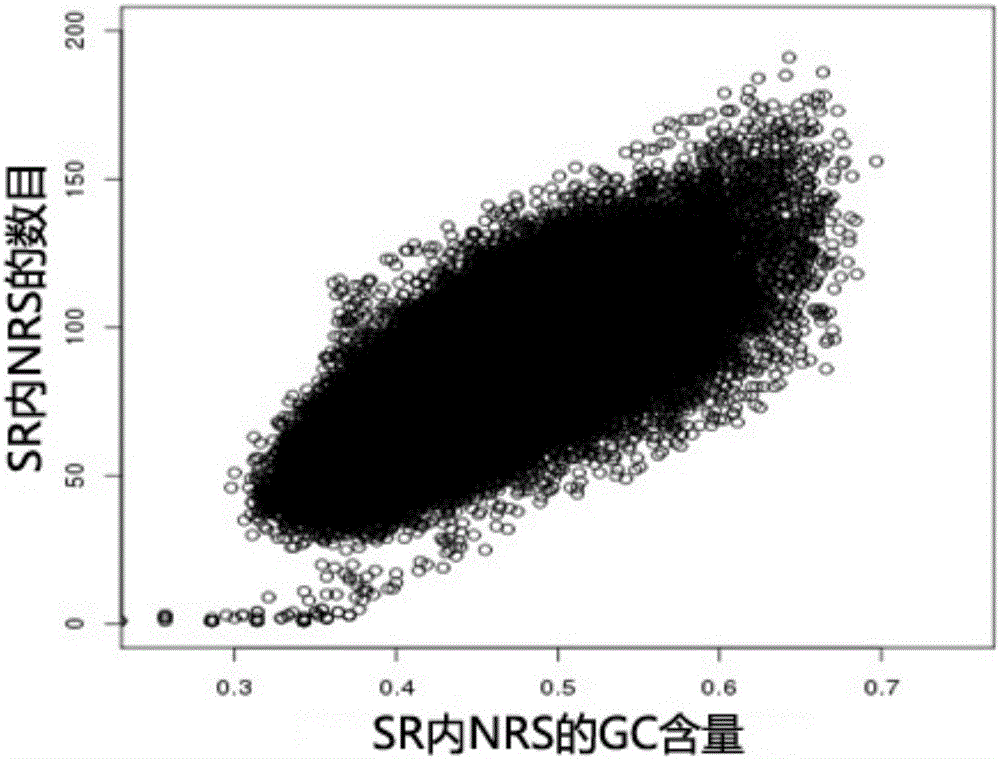
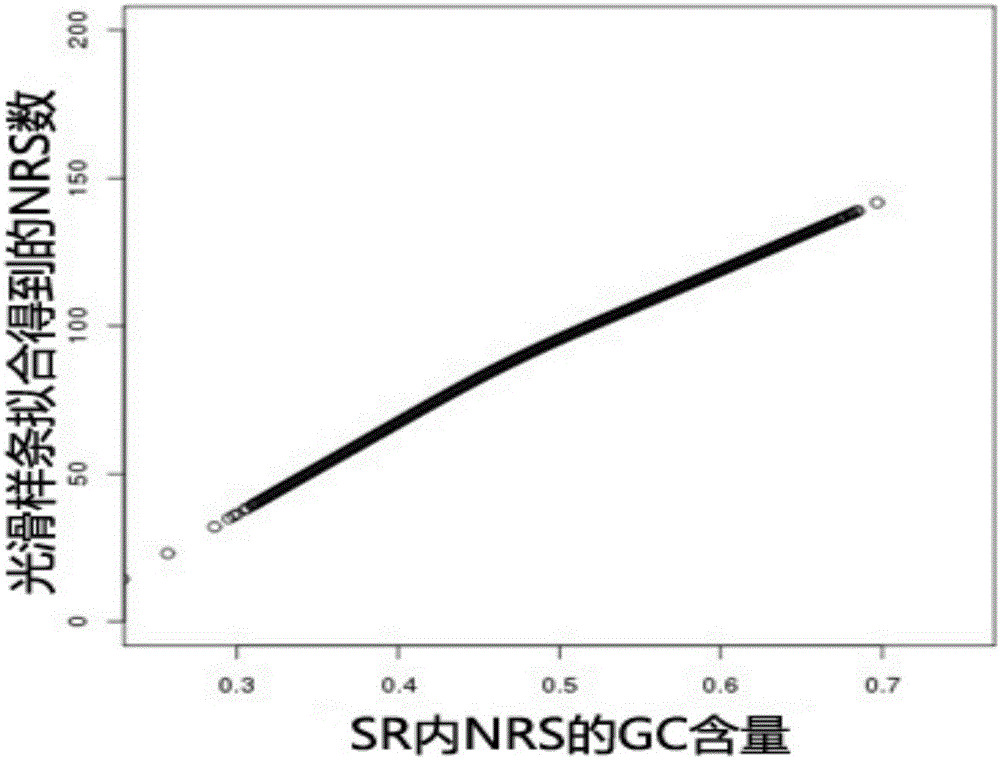
The present invention provides a processing method and processing apparatus for sequencing data. The processing method comprises: acquiring nucleotide sequence information originating from a maternal peripheral blood sample by means of high throughput sequencing; dividing a reference genome into a plurality of specific regions, wherein NRSc values in the specific regions are equal; allocating nucleotide sequence information originating from all chromosomes of the maternal peripheral blood sample to the plurality of specific regions of the reference genome, and collecting statistics of an NRSc value of the sample in each specific region; modifying the NRSc value of the sample in each specific region by using a GC content, and marking a modified NRSc value as an NRSs' value; based on the NRSs' value, separately collecting statistics of mean values of NRSs' values of all specific regions on a target chromosome and a contrast chromosome, and marking the mean values as a first mean value and a second mean value; and performing a difference check on the first mean value and the second mean value, and according to a difference check result, determining whether the chromosome has aneuploidy. The processing method improves accuracy of sequencing data processing.
Owner:GUANGZHOU RIBOBIO
Reaction system and kit for detecting African swine fever virus nucleic acid and application thereof
PendingCN111254223AReal-time detectionImprove featuresMicrobiological testing/measurementMicroorganism based processesAfrican swine fever virusEnzyme
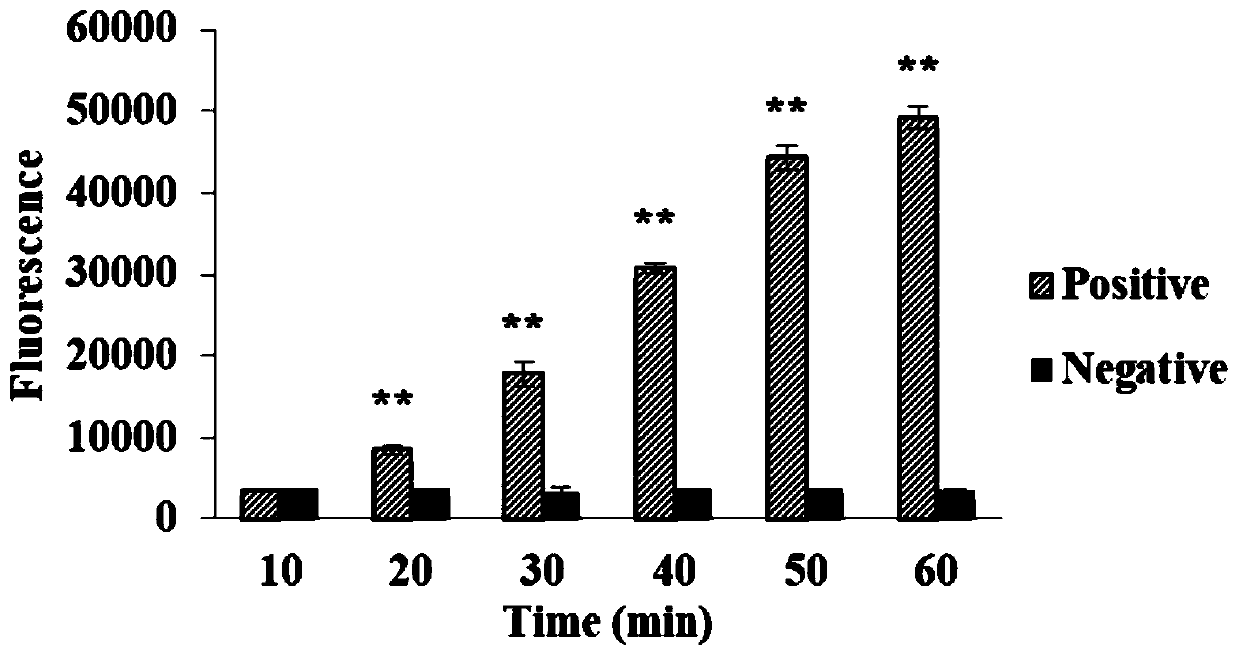
The invention relates to a reaction system for detecting African swine fever virus nucleic acid. The reaction system is an SHERLOCK reaction system. The system comprises an RPA primer pair for amplifying a target nucleic acid fragment and / or crRNA, the crRNA is used for combining ssRNA transcribed by the amplified target nucleic acid fragment, the primer pair comprises primers with sequences shownas SEQ ID NO.1 and SEQ ID NO.2, the crRNA is synthesized from crDNA, and the sequence of the crDNA is shown as SEQ ID NO.3. The system further comprises Cas13a, a fluorescence labeling probe and thelike, the Cas13a is combined with the crRNA combined with the ssRNA so as to have RNA enzyme activity, the Cas13a with the RNA enzyme activity cuts the fluorescence labeling probe to generate fluorescence, and the generated fluorescence can be detected in real time. The invention also relates to a kit comprising the reaction system and related application of the RPA primer pair and / or the crRNA. The kit and a detection method are used for detecting the African swine fever virus nucleic acid, can realize constant-temperature detection at 37 DEG C, have short reaction time, high detection speed,and high sensitivity and the specificity.
Owner:PLANTS & ANIMALS & FOOD TESTING QUARANTINE TECH CENT SHANGHAI ENTRY EXIT INSPECTION & QUARANTINE BUREAU
Preparation method and application of cobalt oxide/mesoporous carbon composite material electrochemical sensor
InactiveCN107228889AHigh sensitivityHigh selectivityMaterial electrochemical variablesLinear relationshipNanocomposite
The invention relates to the field of rapid detection of food safety and particularly relates to a preparation method and application of an electrochemical sensor based on a cobalt oxide / mesoporous carbon nanocomposite material and application of the electrochemical sensor in fast detection of nitrite content in a food. The preparation method of the electrochemical sensor comprises preparing a nanocomposite material carrying cobalt oxide particles and graphitized mesoporous carbon, and modifying a work electrode of the electrochemical sensor. The electrochemical sensor can detect a standard solution of nitrite, determine the linear relationship between the nitrite concentration and current, determine nitrite in the food and compare the nitrite content in the food and the linear relationship to obtain nitrite content of the food to be detected. The electrochemical sensor can determine nitrite content of a food, is easy to operate, has a fast detection rate and high detection sensitivity and provides a novel detection method for efficient and rapid detection of nitrite content in a food.
Owner:SHANGHAI OCEAN UNIV
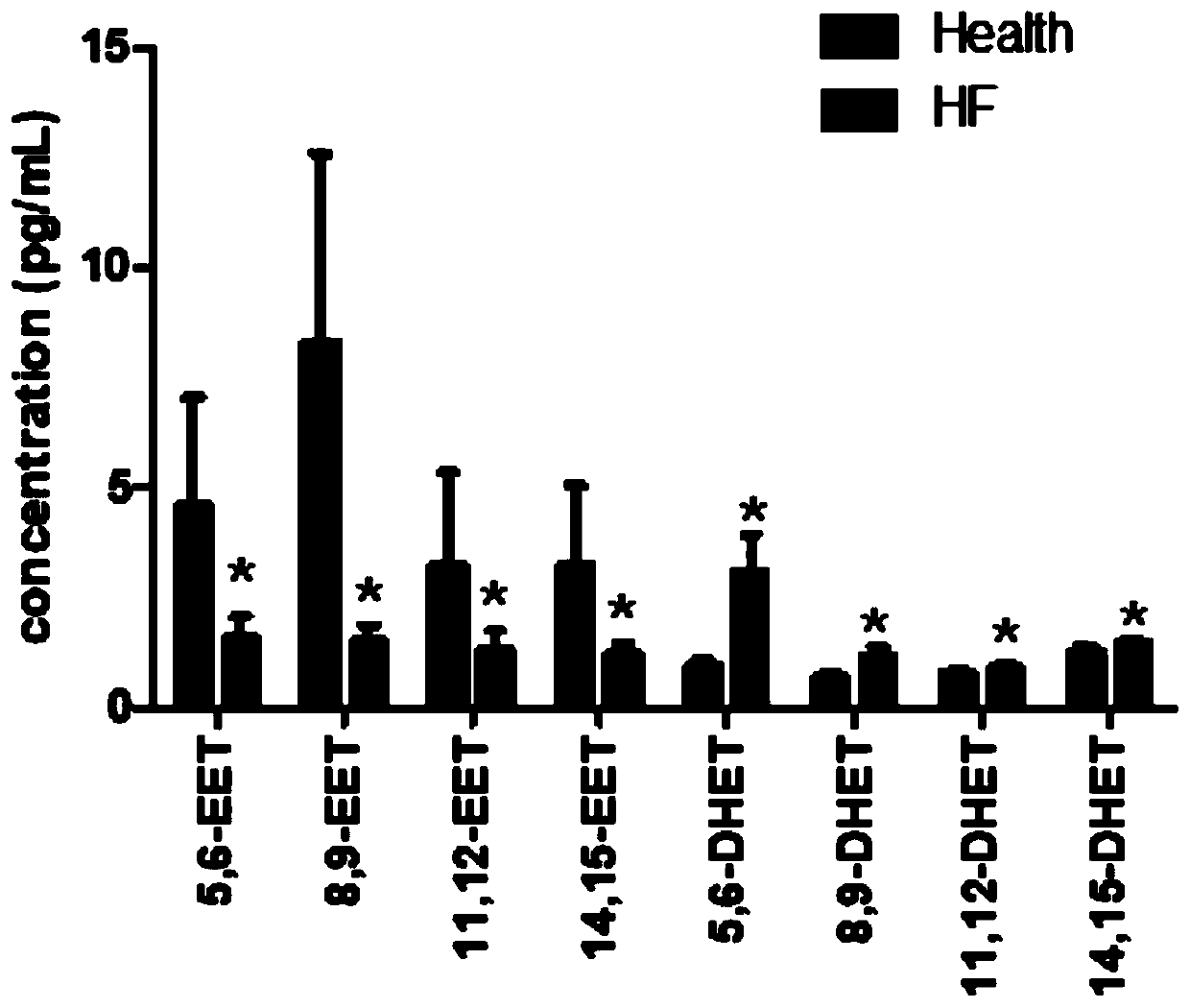
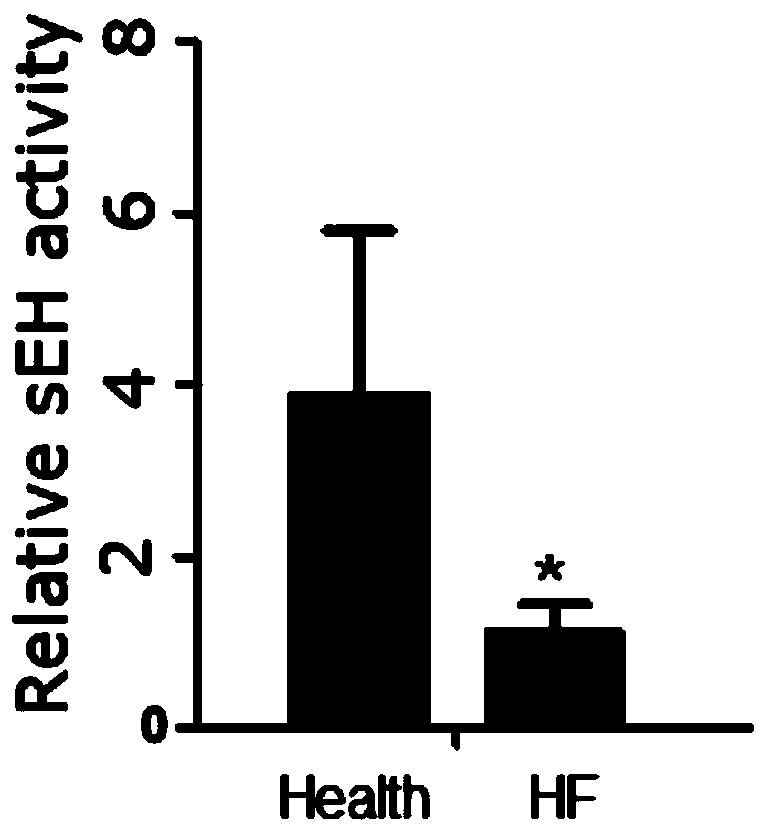
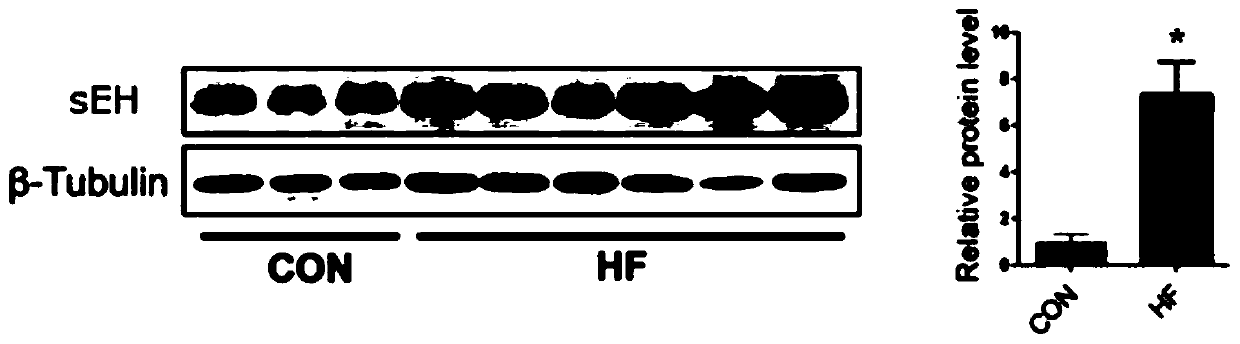
Application of EETs, sEH and sEH inhibitors in chronic heart failure
ActiveCN110161242AEasy diagnosisGood treatment effectComponent separationDisease diagnosisMedicineTreatment field
The invention discloses an application of EETs, sEH and sEH inhibitors in chronic heart failure, and belongs to the field of diagnosis, prevention and treatment of cardiovascular diseases. Two novel biomarkers-EETs and sEH are found, the effect of the biomarkers-EETs and sEH in the diagnosis of biomarkers in the chronic heart failure is proved as well as the effect of the biomarkers-EETs and sEH as a drug target in preventing, alleviating and / or improving the chronic heart failure; therefore, by detecting the EETs and sEH levels, the EETs and the sEH can predict and assist in the diagnosis ofthe chronic heart failure or assess the prognosis of the chronic heart failure; and meanwhile, the EETs level is controlled through drugs, diseases can be subjected to more intensive diagnosis and follow-up treatment, thus the EETs and the sEH haves huge application value in clinical practice.
Owner:TONGJI HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI TECH
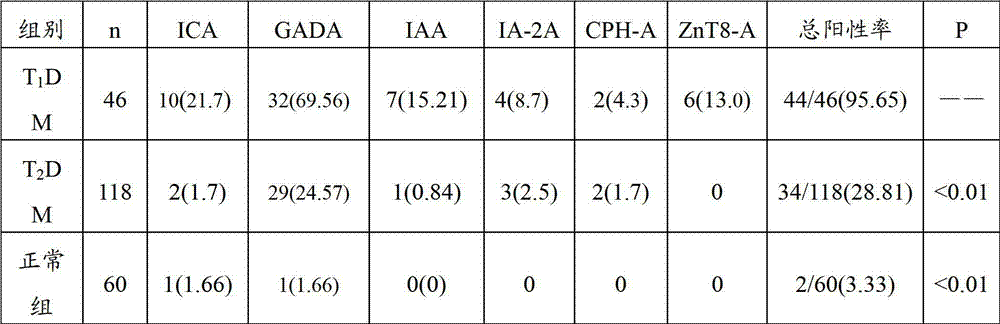
Kit for combined detection of 6 diabetic antibodies
The invention relates to the technical field of biology, and in particular relates to a kit for combined detection of 6 diabetic antibodies. The kit is used for combined detection of 6 antibodies such as ICA, GADA, IAA, IA-2ACPH-A and ZnT8-A in T1DM, T2DM and serum of a normal person, has high sensitivity (98 percent) and specificity (99.6 percent) for I type diabetic diagnosis, and provides efficient and fast detection means for classificatory diagnosis of diabetes.
Owner:SHENZHEN BLOT BIOTECH
Homocysteine detection kit and preparation method thereof
The invention relates to the technical field of biology, in particular to a homocysteine detection kit and a preparation method thereof. According to the method, the traditional colloidal gold is replaced by novel fluorochrome quantum dot, and homocysteine in human serum can be accurately, quantitatively, rapidly and sensitively detected. The instrument required by the kit is different from the previous large expensive instrument in a clinical laboratory, only a small fluorescence quantitative analyzer is required, and real-time detection is realized.
Owner:SHENZHEN BLOT BIOTECH
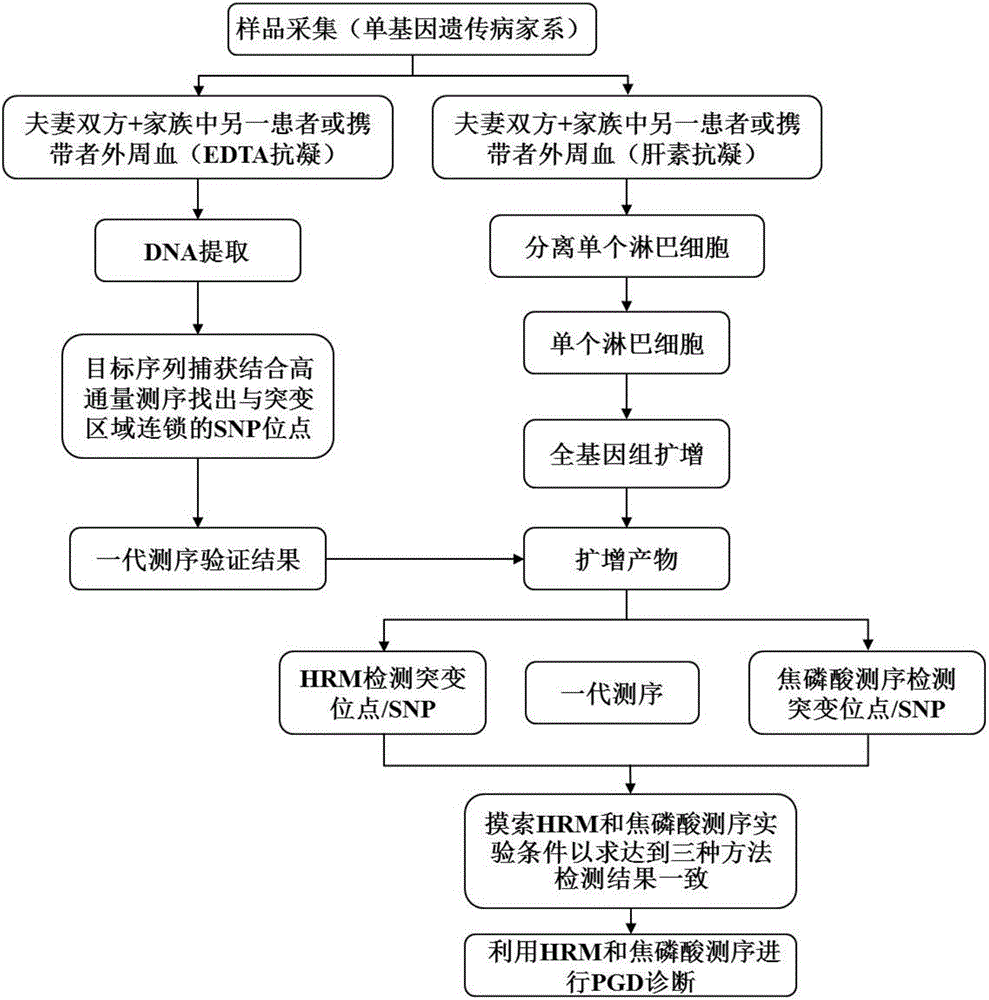
Method for genetic testing in single cells by HRM (high resolution melting) and pyrosequencing
InactiveCN106636435APrevent diseaseImprove population qualityMicrobiological testing/measurementDisease familyMelting curve analysis
The invention belongs to the technical field of medical science and discloses a method for genetic testing in single cells by HRM (high resolution melting) and pyrosequencing. By high-throughput sequencing for haplotype analysis of a single-gene inheritance disease family, an analysis method for mutation detection and SNP (single nucleotide polymorphism) by adoption of a single-cell whole genome amplification product as a template on the basis of HRM and pyrosequencing is established. The method can be applied clinically directly to provide fast detection means for PGD (preimplantation genetic diagnosis) diagnosis of patients suffering from single gene inheritance diseases.
Owner:ZHEJIANG UNIV
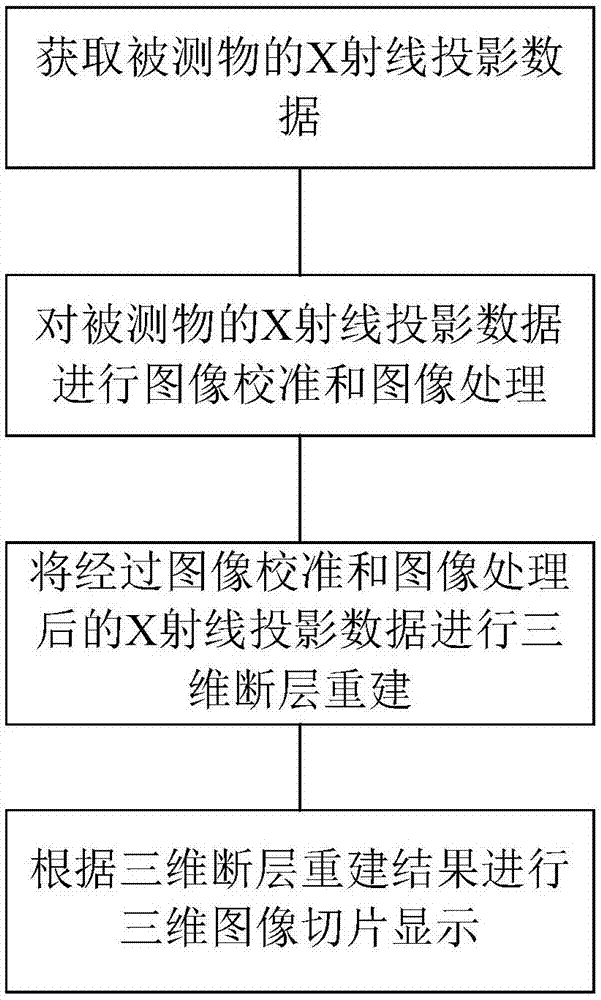
X-ray detection technology-based three-dimensional tomographic reconstruction and slice display method
The present invention discloses an X-ray detection technology-based three-dimensional tomographic reconstruction and slice display method. The method comprises the following steps that: (1) X-rays are adopted to scan a detected object, the X-ray projection data of the detected object are obtained; (2) image calibration and image processing are performed on the X-ray projection data; (3) three-dimensional tomographic reconstruction is performed on the X-ray projection data which have been subjected to the image calibration and image processing; and (4) three-dimensional image slice display is performed according to a three-dimensional tomographic reconstruction result. The method of the invention has the advantages of simple steps and convenient operation. With the method adopted, the internal structure and state of equipment can be reproduced realistically in a three-dimension mode with obtained limited data, and inspectors can be facilitated to directly observe and analyze the state of the equipment.
Owner:ELECTRIC POWER RES INST OF GUANGDONG POWER GRID +1
Beta2-MG chemiluminescence immunoassay kit and preparation method therefor
InactiveCN109239359AGuaranteed SensitivityHigh sensitivityChemiluminescene/bioluminescenceBiological testingBiotin-streptavidin complexMg Antibody
The invention discloses a beta2-MG chemiluminescence immunoassay kit and a preparation method therefor, belongs to the technical field of the immunoassay, and solves the problems of low technique sensitivity and high cost of the prior art. The kit comprises the following reagents: R1: the liquid containing streptavidin-coated magnetic beads; R2: the liquid containing the acridinium ester-labeled beta2-MG antibody; and R3: the liquid containing the biotin-labeled beta2-MG antibody. The beta2-MG chemiluminescence immunoassay kit of the invention has the advantages of high detection sensitivity,strong specificity, good repeatability, wide detection range and good stability, and can be widely used for clinical detection of the beta2-MG.
Owner:DIRUI MEDICAL TECH CO LTD
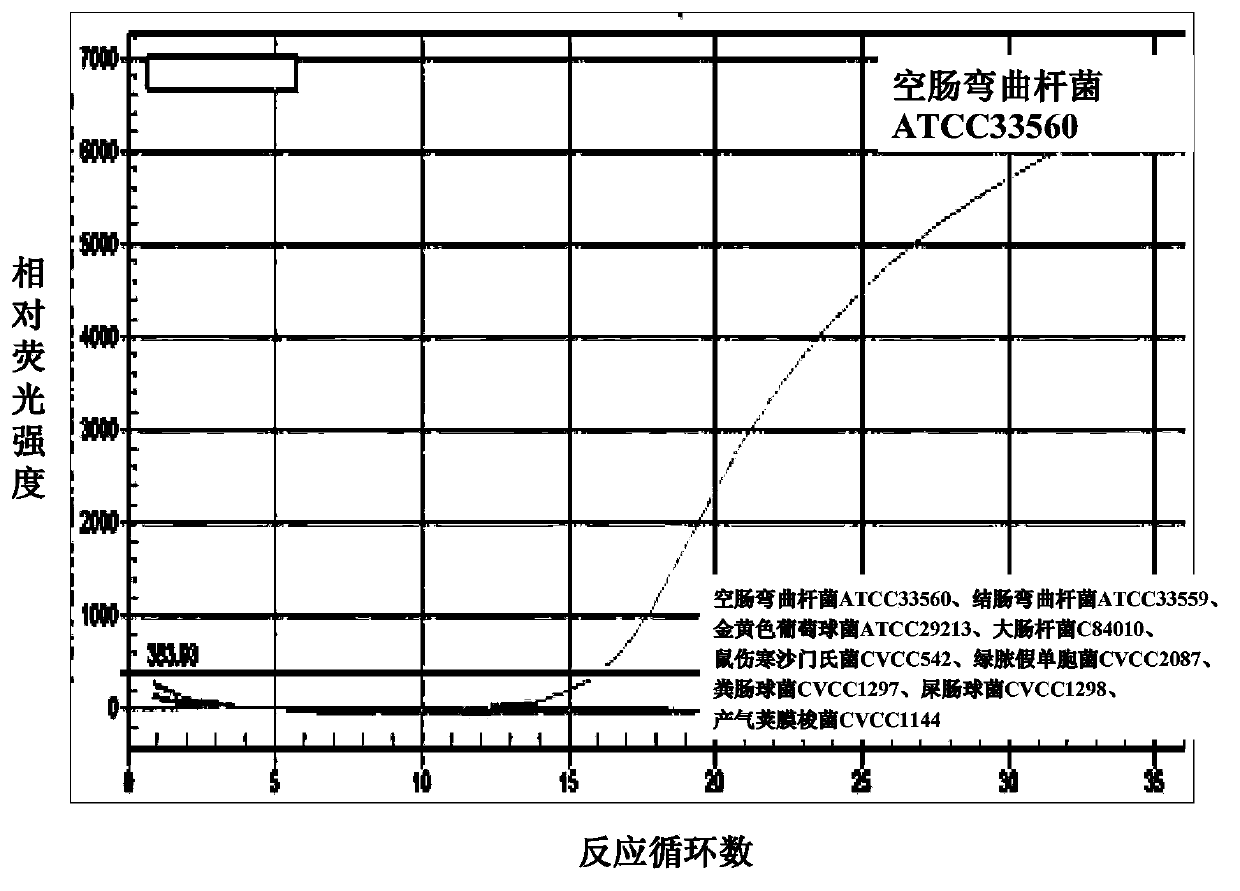
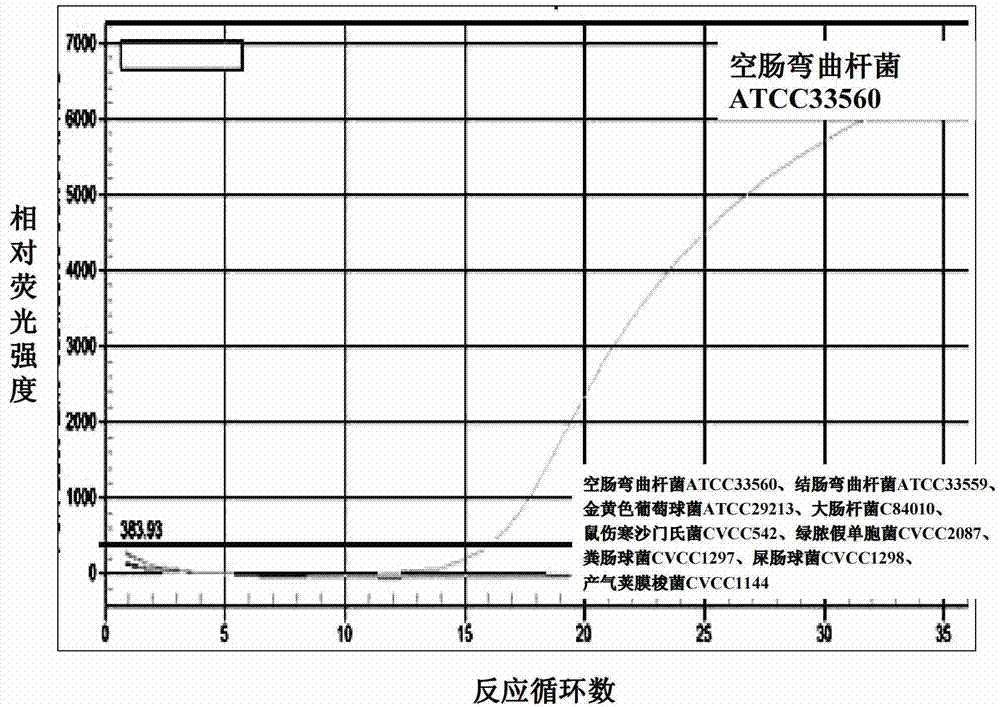
Multiple real-time fluorescent PCR method for identifying the drug-resistant mutation of macrolides and for identifying Campylobacter jejuni
ActiveCN103421892AIncrease hybrid stabilityThe result is accurateMicrobiological testing/measurementFluorescence/phosphorescenceBacteroidesClinical trial
The invention belongs to the field of bacterial drug-resistant molecular detection, and relates to a multiple real-time fluorescent PCR method for identifying the drug-resistant mutation of macrolides and for identifying Campylobacter jejuni. The method is characterized in that an idiocratic primer, MGB probe, and combination of both are designed, not only is the specificity identification performed for the Campylobacter jejuni, but also the mutation of nucleotide of 2074th and 2075th of the 23S rRNA gene of Campylobacter jejuni and that of nucleotide of 170th and 221st of L4 flagellum gene rp1D of ribosome protein can be detected at the same time. The quick detection for drug-resistant mutation points of the various macrolides and the specificity identify for the Campylobacter jejuni are realized, which is first realized in the same reaction system, so that the identification and drug tolerance detection for the Campylobacter jejuni in clinical trials is greatly reduced, and as a result, a novel method is provided for Campylobacter jejuni drug-resistant monitoring and medicines for clinical trials infection treatment.
Owner:HUAZHONG AGRI UNIV
Polymerase chain reaction (PCR) primer pair for identifying H9 subtype avian influenza virus and application thereof
InactiveCN103667519AStrong specificityRapid detection meansMicrobiological testing/measurementDNA/RNA fragmentationLaryngotracheitis virusEpidemiologic survey
The invention discloses a polymerase chain reaction (PCR) primer pair for identifying an H9 subtype avian influenza virus (AIV) and application thereof. The PCR primer pair disclosed by the invention is composed of two single chain deoxyribonucleic acids (DNAs), wherein the two single chain DNAs are single chain DNAs shown in SEQ ID NO:1 and SEQ ID NO:2 in a sequence table. The HA gene of the H9 subtype AIV in a sample can be subjected to specific amplification, and the length of a target segment is 425bp. The method is free of cross reaction on H3, H4, H5 and other subtype AIVs, and a newcastle disease virus, avian infectious bronchitis, an infectious bursal disease virus, an infectious laryngotracheitis virus and the like; the minimum detectable quantity of virus allantoic fluid is 1*10<4.25>EID50 / 100mu L. Compared with the conventional methods such as a hemagglutination inhibition test of the virus and the like, the accordance rate of the identification result is 100%. A rapid, specific and sensitive detection means is provided for identification of the H9 subtype AIV. The PCR primer pair can be applied to rapid diagnosis of a disease caused by the H9 subtype AIV, and has a good application prospect in the aspects of clinical diagnosis and epidemiological investigation.
Owner:LIAOCHENG UNIV
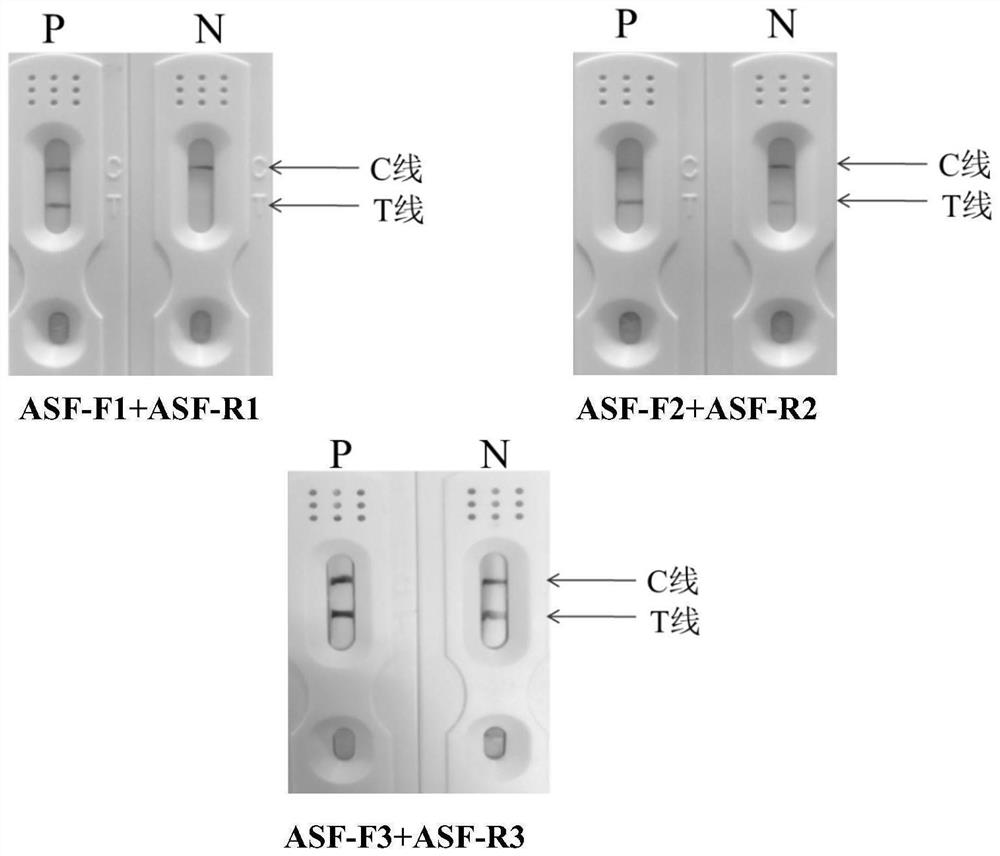
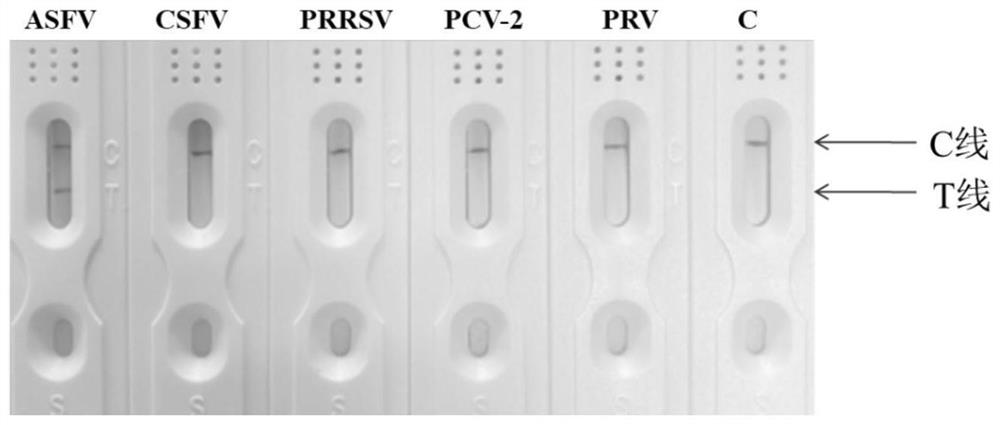
African swine fever virus nucleic acid rapid detection kit and application
InactiveCN112746134AStrong specificityShort detection timeMicrobiological testing/measurementDNA/RNA fragmentationClassical swine fever virus CSFVAfrican swine fever virus
The invention provides an African swine fever virus nucleic acid rapid detection method and kit, and belongs to the technical field of biology. The kit provides specific RPA labeled primers ASF-F and ASF-R for detecting nucleic acid of the African swine fever virus, and the sequences of the specific RPA labeled primers ASF-F and ASF-R are shown as SEQ ID No.1 and SEQ ID No.2. The kit also provides a detection test strip which can be used for detecting RPA amplification products. The RPA primers are adopted to amplify sample DNA respectively, the amplification products are detected through the test strip, and whether the sample is infected with the African swine fever virus or not can be judged. The detection method established aiming at the nucleic acid of the African swine fever virus does not need special equipment, the reaction time is only 25 minutes, the sensitivity is up to 100 cp / microliter, no cross reaction exists in four common swine-origin viruses, and the specificity is high. The African swine fever virus nucleic acid rapid detection kit provides a simple and effective detection means for detecting the nucleic acid of the African swine fever virus, and is suitable for field detection.
Owner:HUAZHONG AGRI UNIV
Primers and reagent kit for rapidly detecting specific vibrio parahemolyticus virulence plasmid pVA1 on site
PendingCN105506142AAccurate methodSimple methodMicrobiological testing/measurementAgainst vector-borne diseasesVibrio parahemolyticusMicrobiology
The invention discloses primers and a reagent kit for rapidly detecting the specific vibrio parahemolyticus virulence plasmid pVA1 on site. The sequences of the primers are shown as SE ID NO:3-6. By the adoption of the primers, the specific vibrio parahemolyticus virulence plasmid pVA1 can be easily, conveniently, rapidly and visually detected, accuracy is high, specificity is good, detection time is short, and operation is easy and convenient.
Owner:海峡两岸农产品检验检疫技术厦门中心
Poria cocos standard decoction UPLC characteristic spectrum construction method and detection method
InactiveCN110441442AQuality improvementAccurate quality controlComponent separationWolfiporia extensaComputer science
The invention relates to a poria cocos standard decoction UPLC characteristic spectrum construction method and a detection method. The poria cocos standard decoction UPLC characteristic spectrum construction method comprises the following steps of (1) precisely weighing poria cocos standard decoction, and preparing to obtain a poria cocos standard decoction supply test solution; and (2) analyzingthe poria cocos standard decoction supply test solution by adopting an ultra-high performance liquid chromatograph to obtain a poria cocos standard decoction UPLC characteristic spectrum. The method is relatively high in precision, stability and reproducibility, fully shows the chemical component characteristics of the poria cocos standard decoction, can comprehensively and accurately control thequality of the poria cocos standard decoction, and also provides a basis for the establishment of the poria cocos medicinal material and poria cocos formula particle quality standard.
Owner:GUANGDONG YIFANG PHARMA
Two-stage cone pulley phase angle error detecting device and detecting method thereof
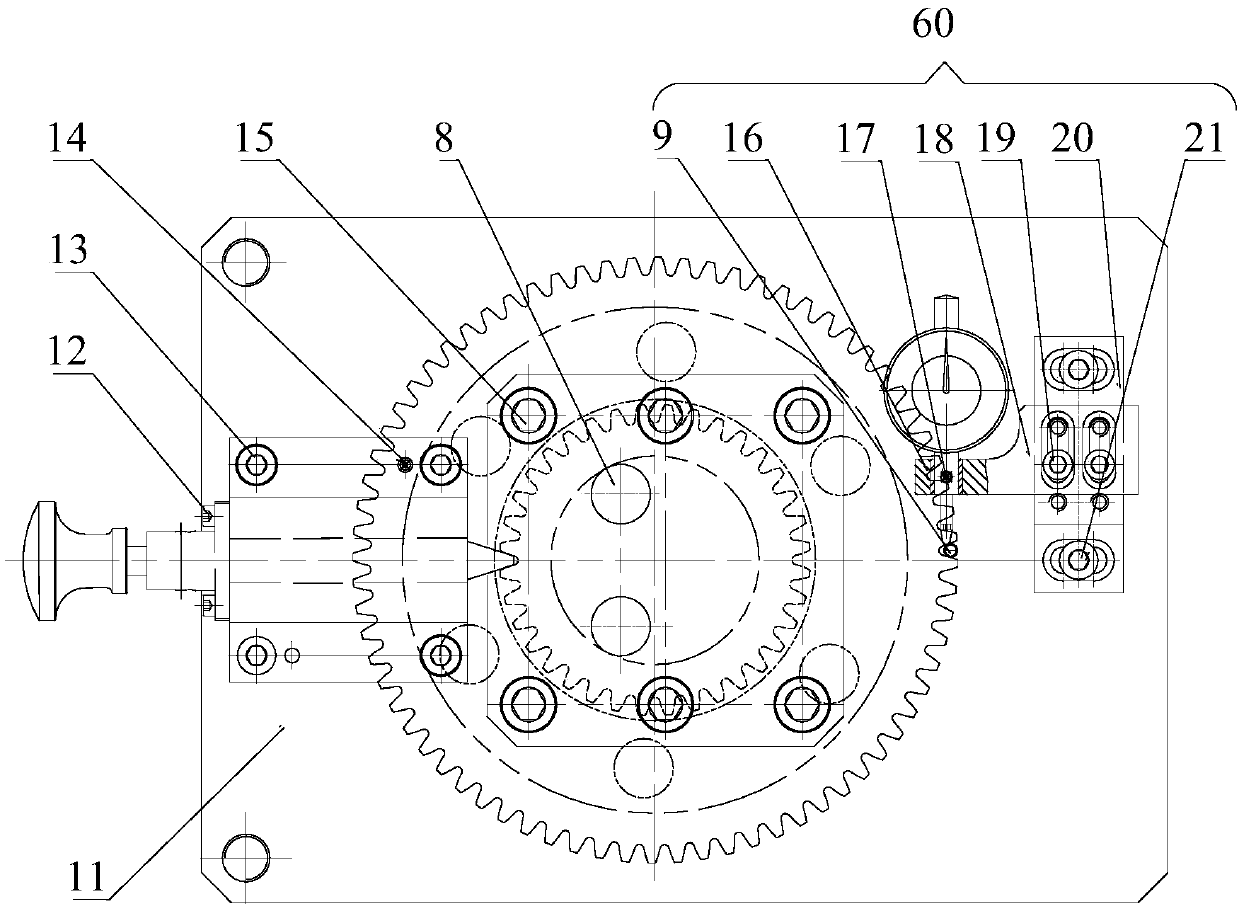
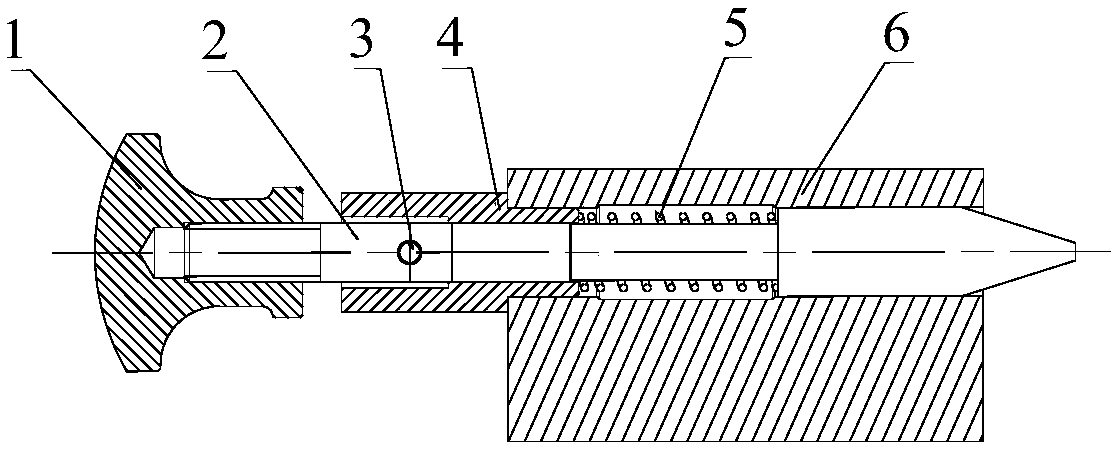
PendingCN109539951AImprove detection efficiencyRapid detection meansAngles/taper measurementsEngineeringPinion
The invention relates to the technical field of two-stage cone pulleys, and discloses a two-stage cone pulley phase angle error detecting device and a detecting method thereof. The device comprises ameasuring base, a two-stage cone pulley centering component, a pinion cogging positioning component and a large gear phase offset distance measuring component. The pinion cogging positioning componentis disposed at the pinion side of the two-stage cone pulley to be detected and radially positions the pinion cogging; the large gear phase offset distance measuring component is disposed on the largegear side of the two-stage cone pulley to measure the offset of the center position of a tooth socket being closet to the axis of the pinion cogging positioning component relative to the corresponding position of a two-stage cone pulley calibration component. The two-stage cone pulley phase angle error detecting device and the detecting method thereof employ the two-stage cone pulley centering component and the pinion cogging positioning component to locate the two-stage cone pulley to be detected to measure the deviation value to obtain an error value so as to greatly improve the detection efficiency of the two-stage cone pulley phase angle.
Owner:DONGFENG MOTOR CO LTD
Rhizoma drynariae crude product and UPLC fingerprint spectrum construction and identification method thereof
The invention relates to a rhizoma drynariae crude product and a UPLC fingerprint spectrum construction and identification method thereof. The construction method comprises the following steps: respectively preparing a reference substance solution and a test substance solution, and detecting the reference substance solution and the test substance solution by adopting a high performance liquid chromatography, wherein the step of preparing the reference substance solution comprises the steps of taking reference substances, adding a solvent and dissolving the reference substances, and the reference substances comprise 5-hydroxymethylfurfural, protocatechuic acid, neoeriocitrin and naringin; and the step of preparing the test solution comprises the following steps: taking a rhizoma drynariae processed product, adding a solvent, extracting, filtering and collecting filtrate, wherein the rhizoma drynariae processed product is a rhizoma drynariae microwave processed product. According to the present invention, the obtained fingerprint can simultaneously contain the characteristic peaks of the rhizoma drynariae crude product and the different processed products thereof, and can completely and comprehensively reflect the chemical component information of the rhizoma drynariae crude product and the different processed products thereof, such that the fingerprint can be simultaneously used for identifying the rhizoma drynariae crude product and the various rhizoma drynariae processed products, and the application range is wide.
Owner:GUANGDONG YIFANG PHARMA
Method for measuring purity of 9-fluorenemethanol
InactiveCN101131377ASimple and accurate measurementRapid detection meansComponent separationOther chemical processesRelative standard deviationPeak area
The invention discloses a purity measuring method of the 9-Fluorenylmethanol. Firstly it confects the marker of purity and the measured sample, then to detect relative chromatogram chart and the peak area using the HPLC; last to converse to 9-Fluorenylmethanol purity of the sample according to the quantity calculation formula of the external scaling method. The invention is first to use the HPLC and the ultraviolet multi-wave detector and the peak is edge and has high separation degree; the separating time is short; the relative standard deviation is 0.25% and the recovery rate is 98.1%-103.2%. The invention can be used to detect the 9-Fluorenylmethanol purity in production, use, the sale and study field.
Owner:BAOSHAN IRON & STEEL CO LTD
Spray deposition amount and coverage area online detection system based on capacitive sensor
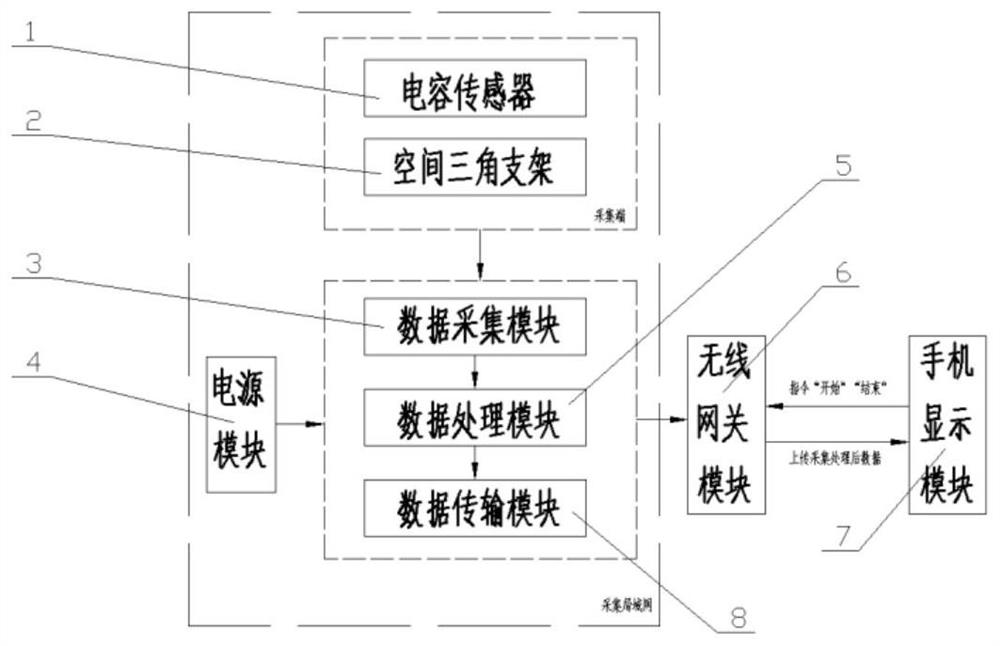
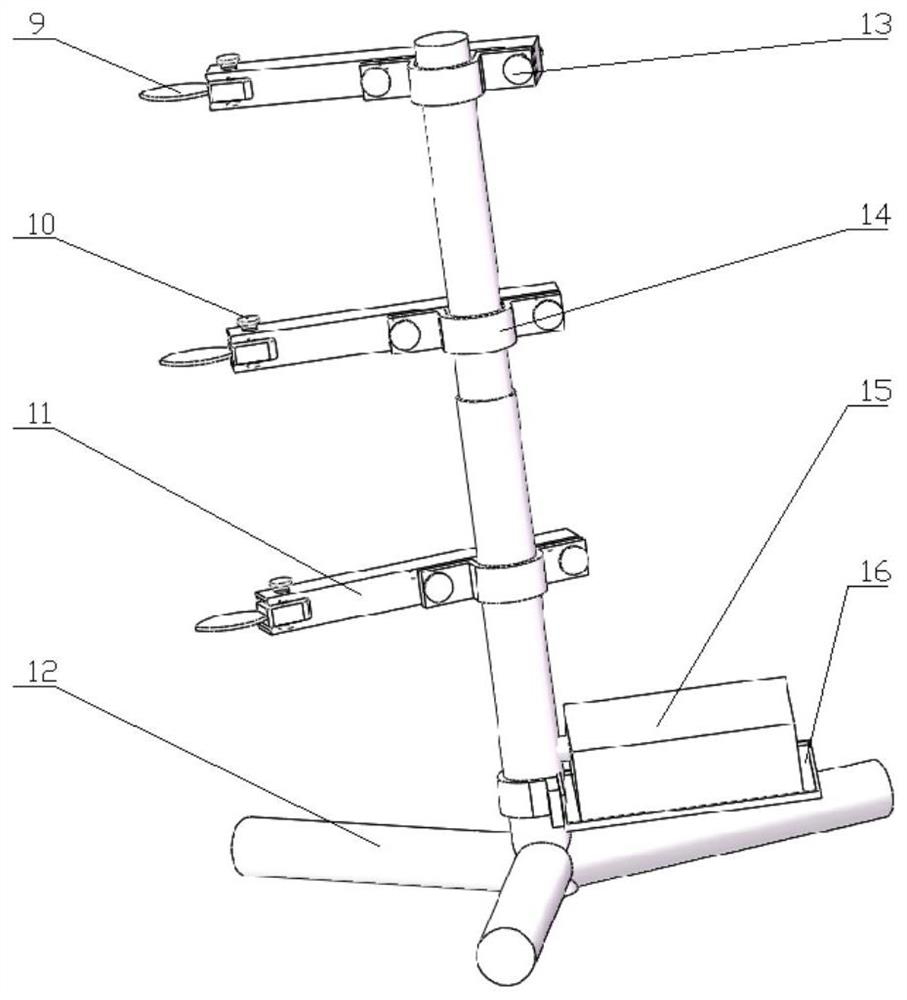
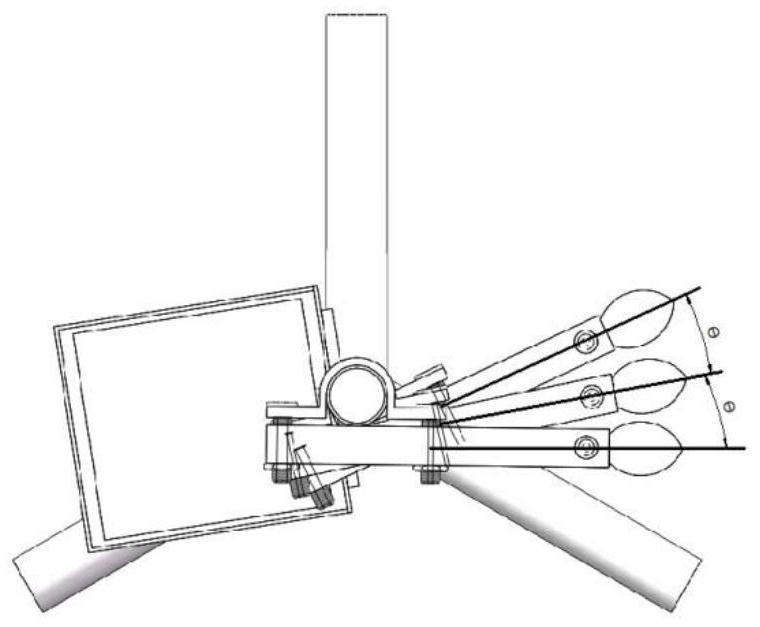
ActiveCN112816372AReal-time measurementOnline Measurement BreakthroughElectric/magnetic area measurementsMaterial analysisSoil scienceAgricultural engineering
The invention discloses a spray deposition amount and coverage area online detection system based on a capacitive sensor, which relates to the field of agricultural intelligent detection and comprises the capacitive sensor, a data acquisition module, a data processing module, a data transmission module, a wireless gateway module, a power supply module, a mobile phone display module and a spatial triangular bracket. The detection system with the function of measuring the space multi-layer spray deposition amount and the coverage area can output the layered spray deposition amount and the coverage area in real time, and the rapid detection and evaluation of distribution of the spray deposition amount and the coverage area are achieved. According to the invention, the workload of traditional measurement of the spray deposition amount and the coverage area can be reduced, and a rapid detection means is provided for basic research of the spray deposition amount and the coverage area.
Owner:JIANGSU UNIV
Fluorescent probe for detecting and quantifying signal molecule of Gram negative bacteria colony
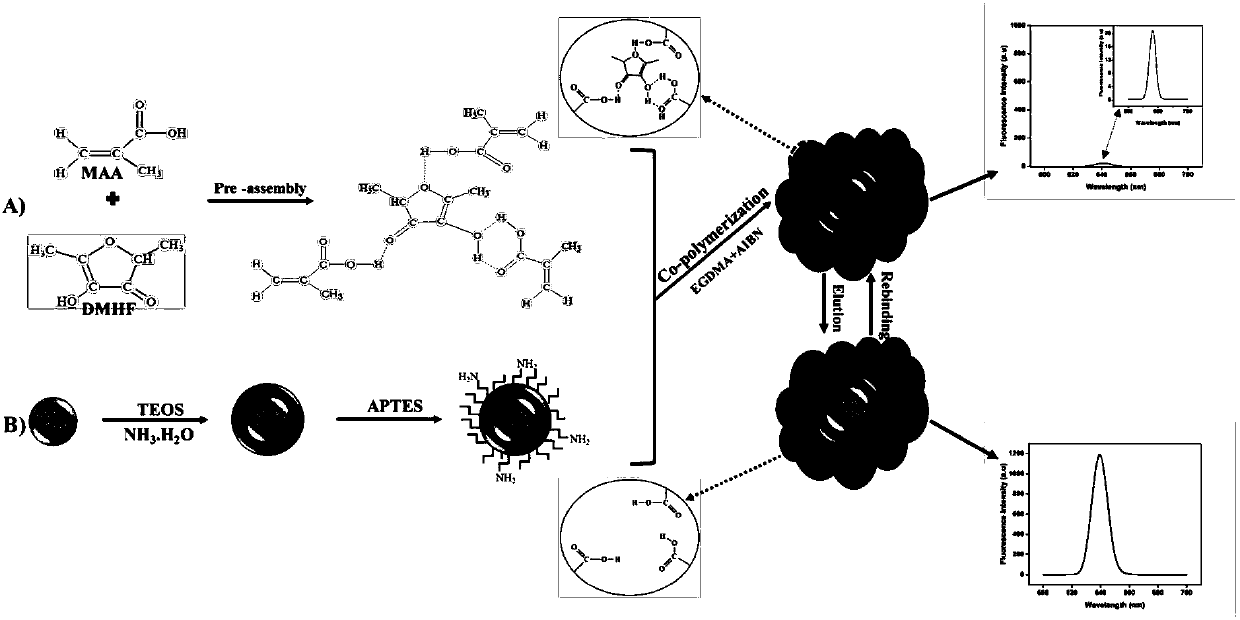
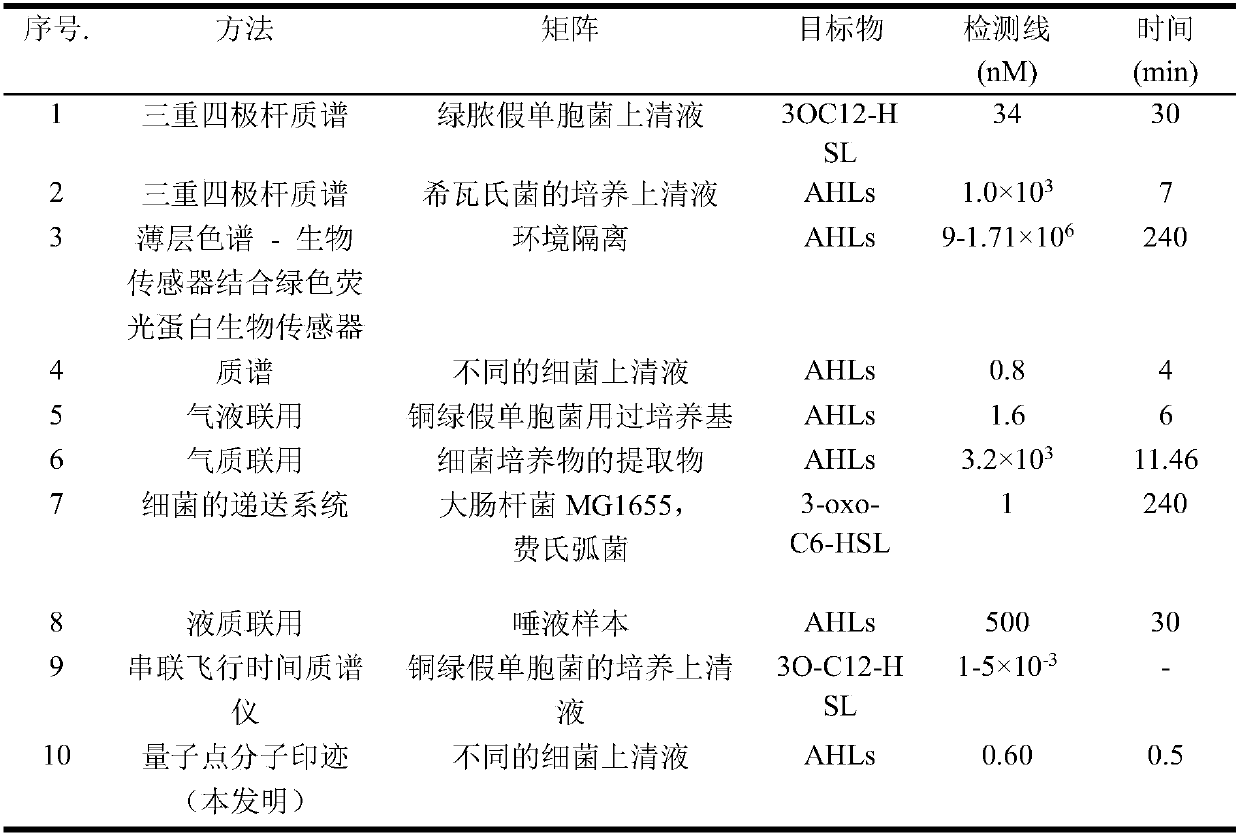
InactiveCN108051419AStrong specificityHigh sensitivityFluorescence/phosphorescenceFluorescenceQuantum dot
The invention discloses a fluorescent probe for detecting and quantifying signal molecule of Gram negative bacteria colony, and belongs to the technical field of rapid detection of pathogenic bacterium. According to the fluorescent probe, a quantum dot is connected with MIP through a surface molecular imprinting technology, and the purpose of accurate quantitative detection is achieved through a fluorescence value on the basis of high degree specificity detection and fluorescence. MIP polymer is precipitated and polymerized on the surface of an amino-silane functional group modified by a CdSe / ZnS quantum dot in advance, so that QDs@MIP is successfully synthesized. The invention further discloses a preparation method and the application of the molecular imprinting fluorescent probe. The fluorescent probe can realize indirect detection of part of Gram negative bacteria, has the advantages of sensitivity, speediness and high specificity, and is low in price, and the method has an important implication for food safety or health care facilities, and can be applied to the direct monitoring of Gram negative bacteria.
Owner:JIANGNAN UNIV
Method and system for detecting linearity and rigidity of bridges and tunnels on basis of fiber-optic gyroscope technology
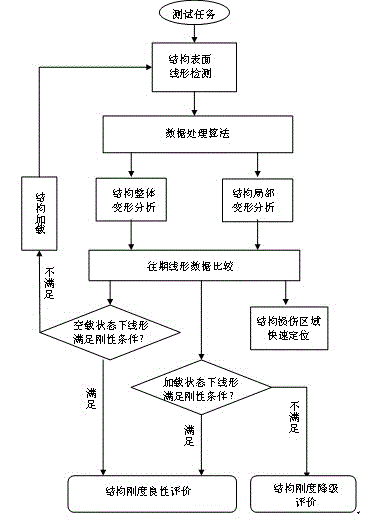
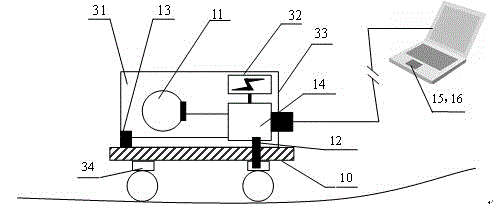
ActiveCN102661716BEasy to catchShort test cycleStructural/machines measurementUsing optical meansOutlier eliminationCorrection algorithm
A detection method and system for alignment and stiffness of bridges and tunnels based on fiber optic gyroscope technology. In this method, the linear detection walking device moves along the inner surface of the bridge or tunnel structure under test, and the data measured by the fiber optic gyroscope and the linear velocity sensor are processed by algorithm, and the motion track of the device is calculated according to the formula, that is, the gravity of the bridge structure under test Direction and continuous deformation trajectory of tunnel strike or cross-section circumferential direction. Based on the data stability judgment and outlier elimination algorithm, the reverse correction algorithm of the position information of the reference point and the reference point, and the filtering algorithm of the overall large deformation of the structure and the local small deformation of the surface, the data is corrected and calculated to obtain the local minimum of the stiffness analysis of bridges and tunnels. Deformation culling curves. This method is used to detect the local damage of bridges and tunnel structures, and the global deformation trajectory detection of bridge vertical, tunnel direction and multi-cross sections. Compared with traditional bridge and tunnel alignment detection, this method has shorter test period, high operability, low cost, high precision and high data consistency.
Owner:WUHAN UNIV OF TECH
Primer for detecting porcine circovirus 3, PCR (polymerase chain reaction) kit and application
InactiveCN107828916AQuick checkAccurate detectionMicrobiological testing/measurementDNA/RNA fragmentationAgricultural sciencePcr method
The invention discloses a primer for detecting porcine circovirus 3, a PCR (polymerase chain reaction) kit and an application. The sequence of an upstream primer of the primer is 5' CGGGAAATCTGACTGAAGTT 3', and the sequence of a downstream primer of the primer is 5' ACTCCTCCGGTACAACATTA 3'. The primer has the advantages that the primer is a pair of specific primers designed for PCV3 (porcine circovirus 3) according to the sequence of the PCV3 in a GenBank, a method for rapidly and accurately detecting the PCV3 is built, strong in specificity, high in sensitivity and good in repeatability, anda rapid and accurate detection means is provided for laboratory diagnosis of the PCV3 and survey of the prevalence state of the PCV3 in Chinese swine herds.
Owner:SHANDONG NEW HOPE LIUHE GROUP
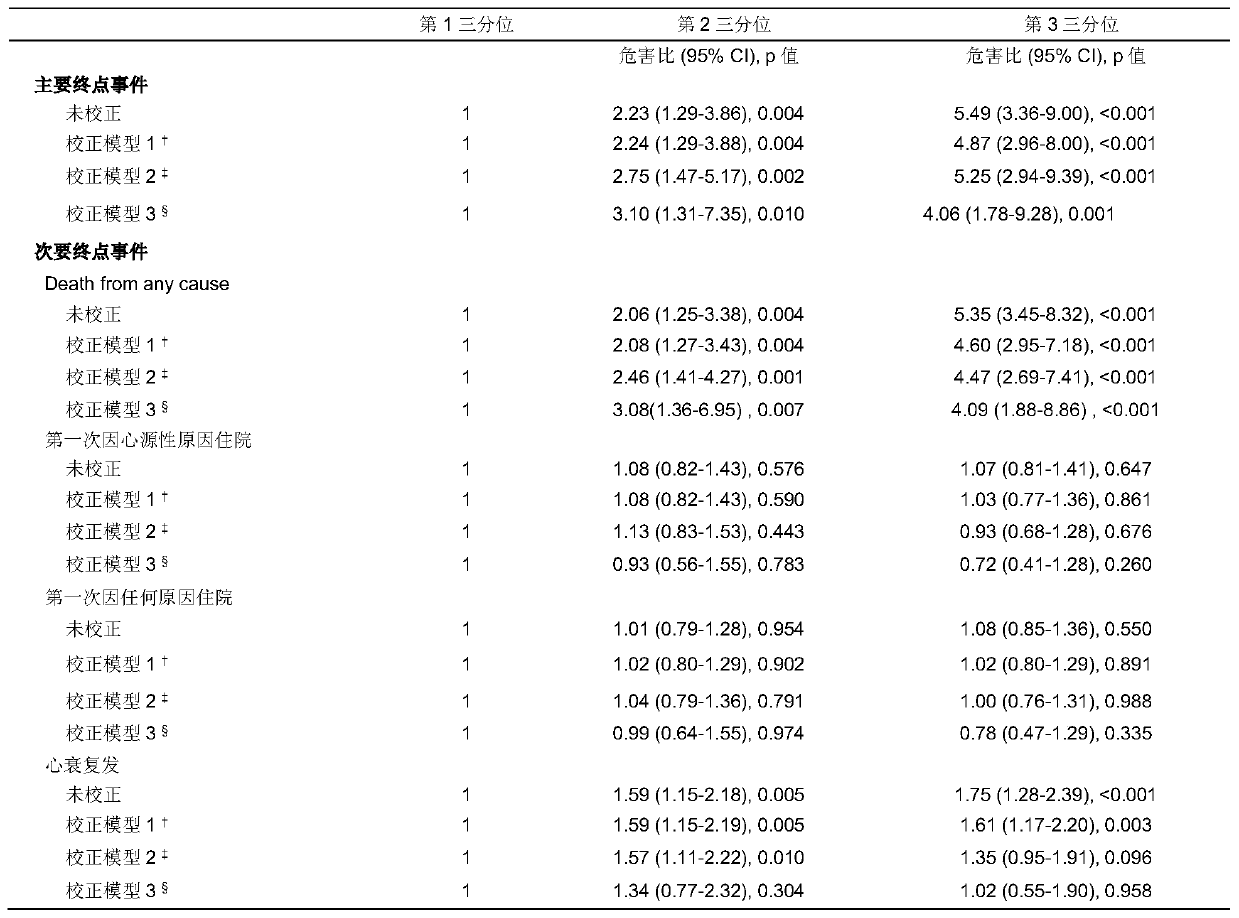
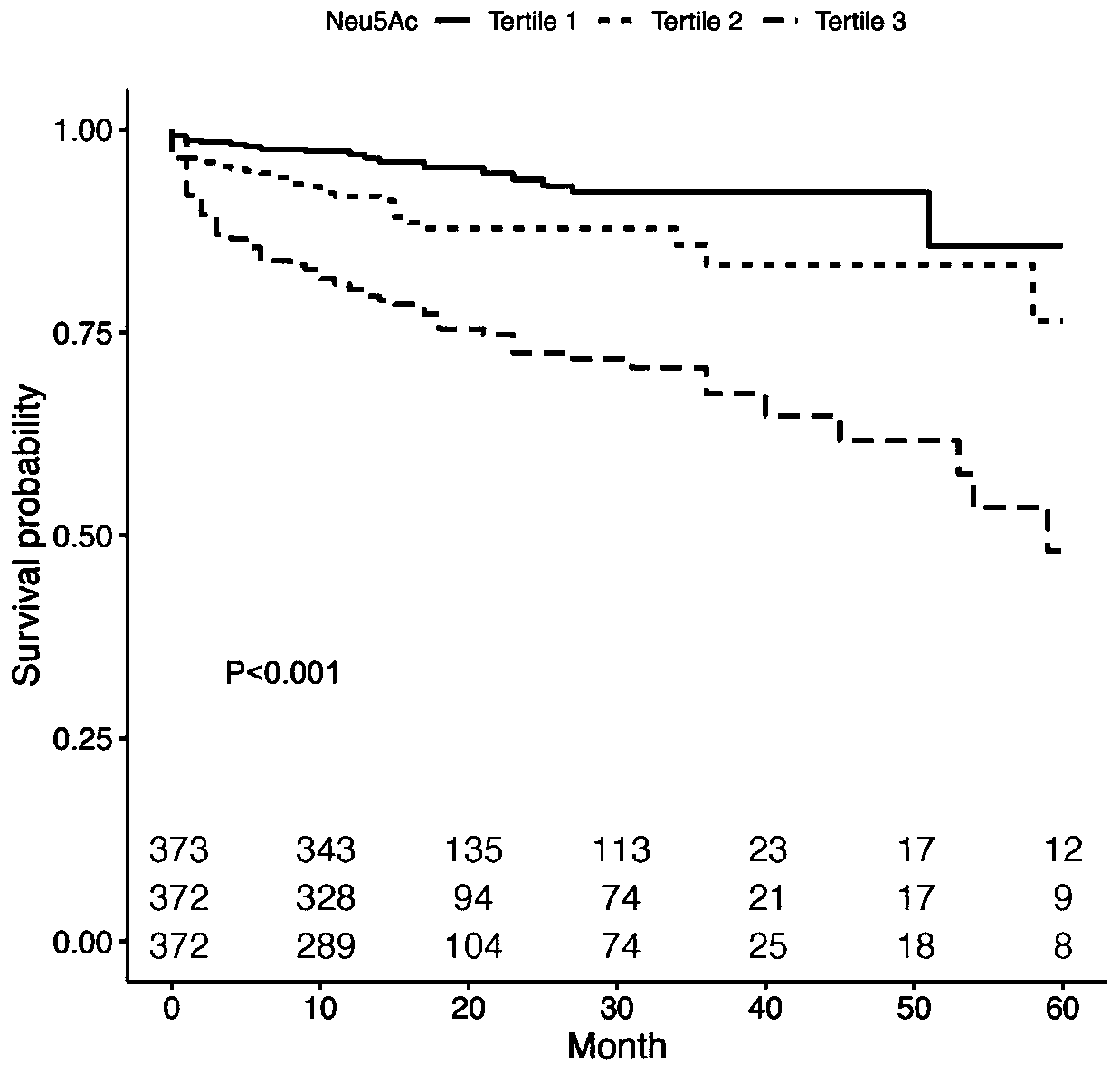
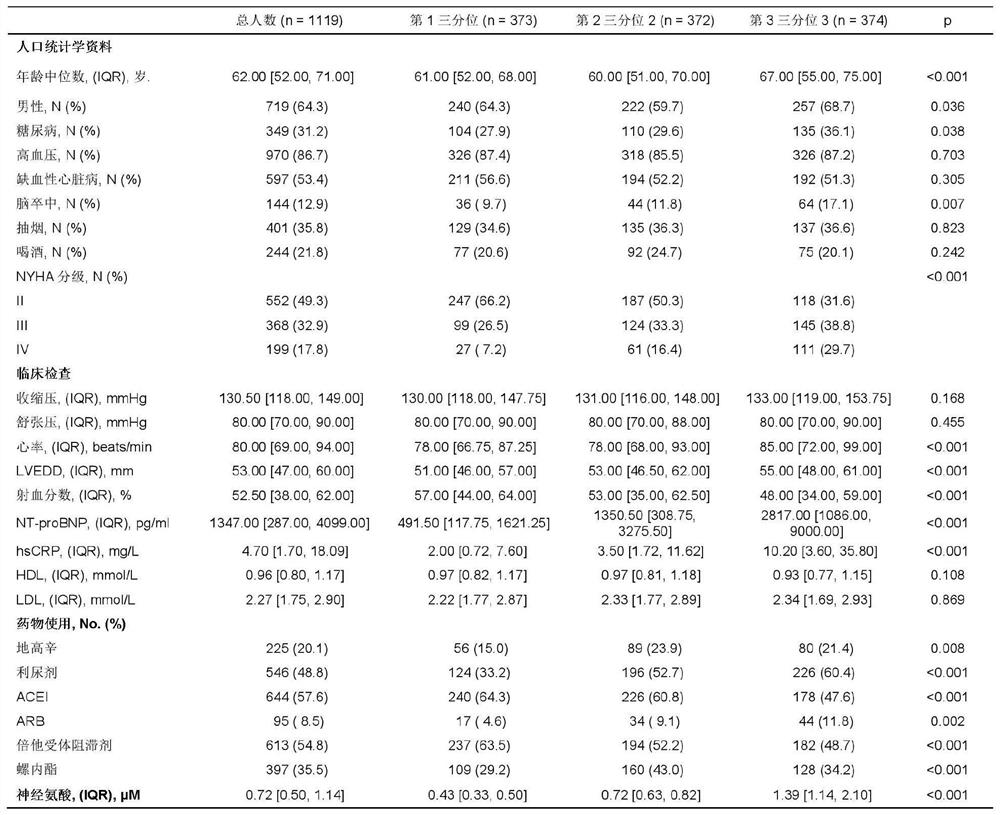
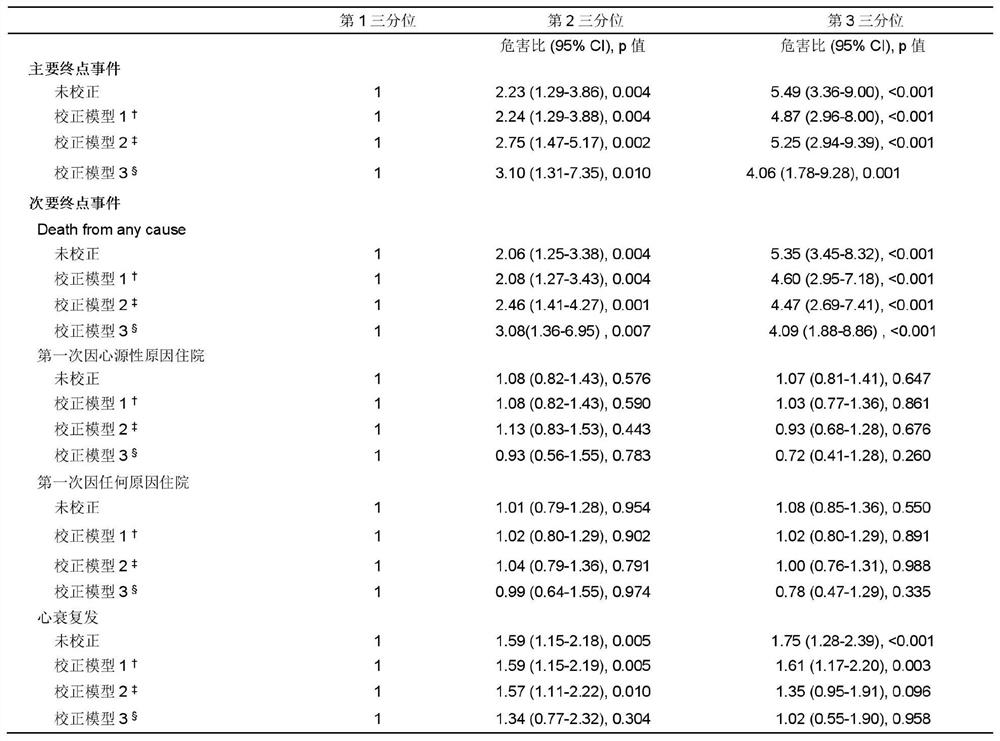
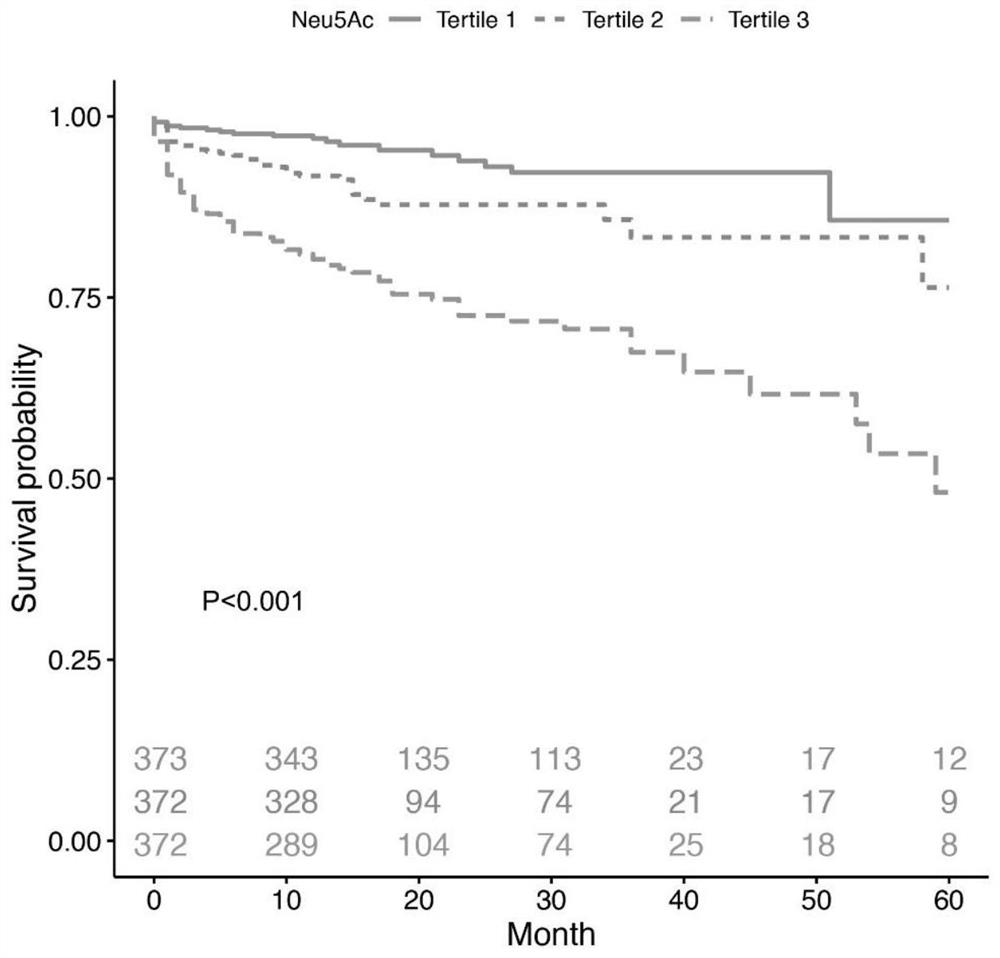
Application of neuraminic acid and neuraminidase inhibitor in chronic heart failure
ActiveCN109799330AEasy diagnosisGood treatment effectOrganic active ingredientsBiological testingPrognosis biomarkerNeuraminic acid
The invention discloses application of neuraminic acid and a neuraminidase inhibitor in chronic heart failure, belongs to the field of diagnosis, prevention and treatment of cardiovascular diseases, discloses a novel biomarker-neuraminic acid, and proves that neuraminic acid plays a role in diagnosis, prognosis biomarkers and risk stratification in the chronic heart failure and a role in preventing, relieving and / or improving the chronic heart failure as a drug target. Therefore, detection of the level of the neuraminic acid by the kit can be used for predicting and assisting in the diagnosisof the chronic heart failure or evaluating the prognosis of the chronic heart failure, and the level of the neuraminic acid is controlled through drugs so as to carry out intensive diagnosis and follow-up treatment on diseases, a favorable course of disease is obtained, and the clinical application value is huge.
Owner:TONGJI HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI TECH
Method for detecting mononucleotide polymorphism by conformational difference gel electrophoresis
InactiveCN102766684ALow costSimple and fast operationMicrobiological testing/measurementNucleotideGel electrophoresis
A method for detecting mononucleotide polymorphism by conformational difference gel electrophoresis. The method comprises the following specific steps: (1) collecting samples; (2) extracting DNA of a sample genome; (3) selecting single nucleotide polymorphisms (SNPs) associated with disease occurrence, drug efficacy and toxicity for a polymerase chain reaction (PCR ) amplification; (4) carrying out conformational difference gel electrophoresis analysis on PCR products, and determining different genotypes according to position and number of bands; and (5) carrying out DNA sequencing on PCR products of samples with different band types, and determining specific genotypes of different samples. According to the invention, the analysis of SNPs is analysis of double-stranded PCR products, without requiring endonuclease; samples can be loaded directly for gel electrophoresis without any prior processing; and no expensive equipment or reagent is required; therefore the method has advantages of simple operation and low cost. This invention provides a fast, accurate, simple, and low-cost detection means for SNPs related to disease susceptibility and individualized drug therapy, and lays foundation for clinical application of the method.
Owner:NANCHANG UNIV
Application of biomarker combination in preparation of diagnostic reagent for fulminating myocarditis and medicine for fulminating myocarditis
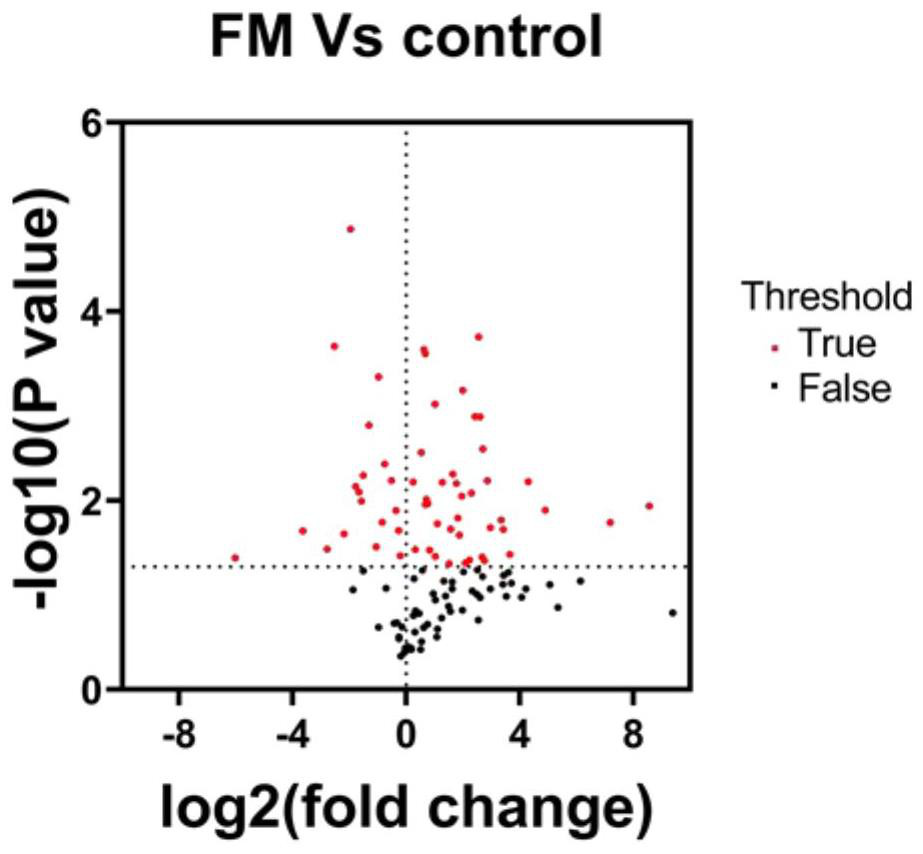
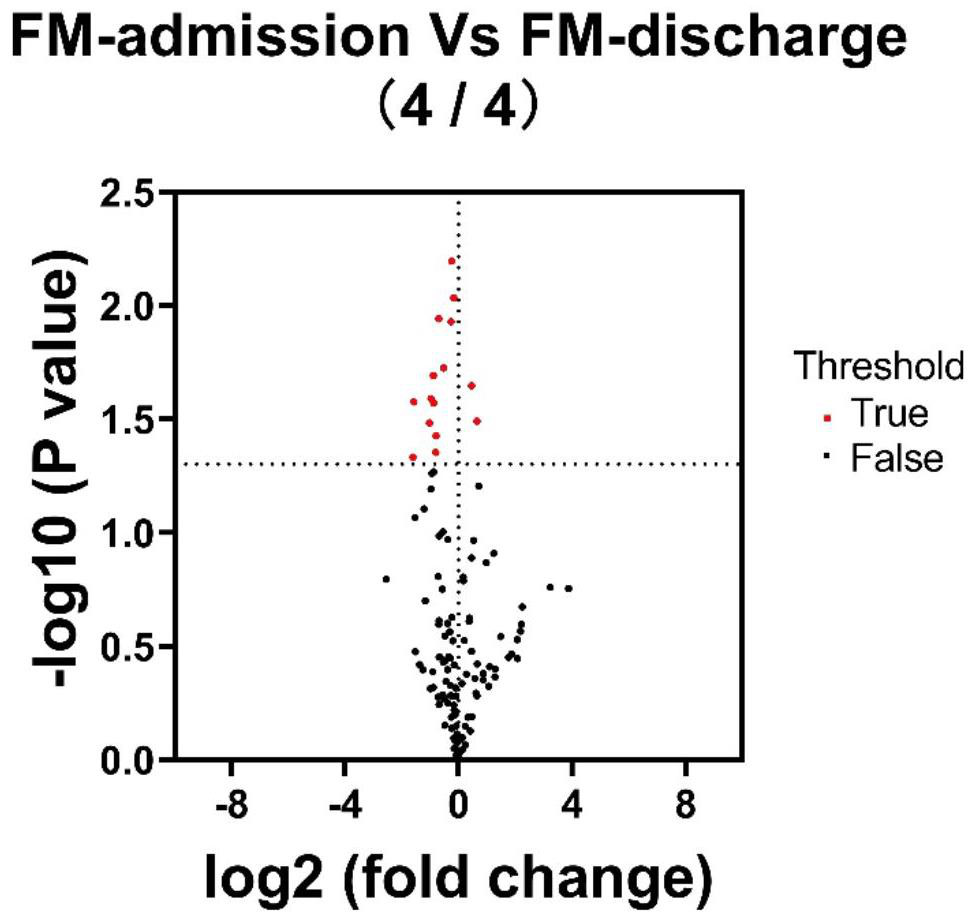
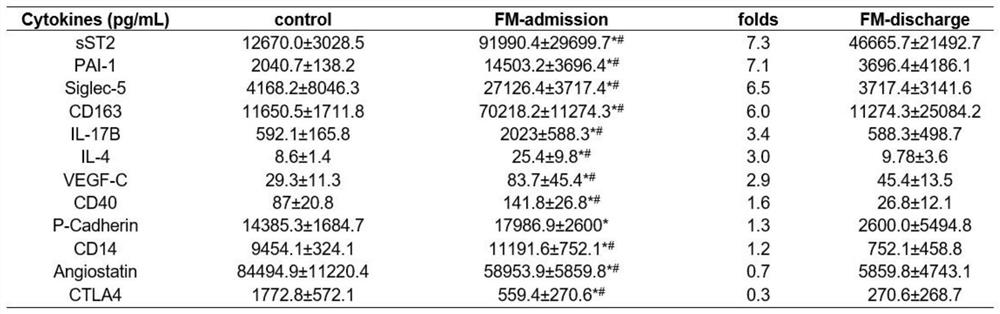
ActiveCN114414812AEasy diagnosisImprove efficiencyDisease diagnosisIndividual particle analysisDiseaseMyocarditis
The invention relates to application of a biomarker composition in preparation of a diagnostic reagent for fulminating myocarditis and a drug for fulminating myocarditis, belonging to the field of disease diagnostic reagents and drugs. The application is characterized in that the biomarker combination comprises Siglec-5, Siglec-5, Siglec-5, Siglec-5, Siglec-5 and Siglec-5, the combination of biomarkers is further selected from the group consisting of sST2, PAI-1, Siglec-5, CD163, CD40, P-Cadherin, CD14, and CTLA4, and the combination of biomarkers is selected from the group consisting of sST2, PAI-1, Siglec-5, CD163, CD40, P-Cadherin, CD14, and The invention finds that the expression of the biomarker in the plasma of a patient with the outbreak myocarditis is increased, and proves that the biomarker plays a role in diagnosing the outbreak myocarditis and the role of the biomarker as a drug target in relieving and / or improving the outbreak myocarditis, and the level of the biomarker can be detected, so that the application of the biomarker in the diagnosis of the outbreak myocarditis and the application of the biomarker in the treatment of the outbreak myocarditis can be realized. The biomarkers can be used for predicting and assisting in diagnosing the outbreak myocarditis or evaluating prognosis of the outbreak myocarditis, meanwhile, the level of the biomarkers is controlled through drugs, more intensified diagnosis and follow-up treatment can be carried out on diseases, and the biomarkers have huge clinical application value.
Owner:TONGJI HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI TECH
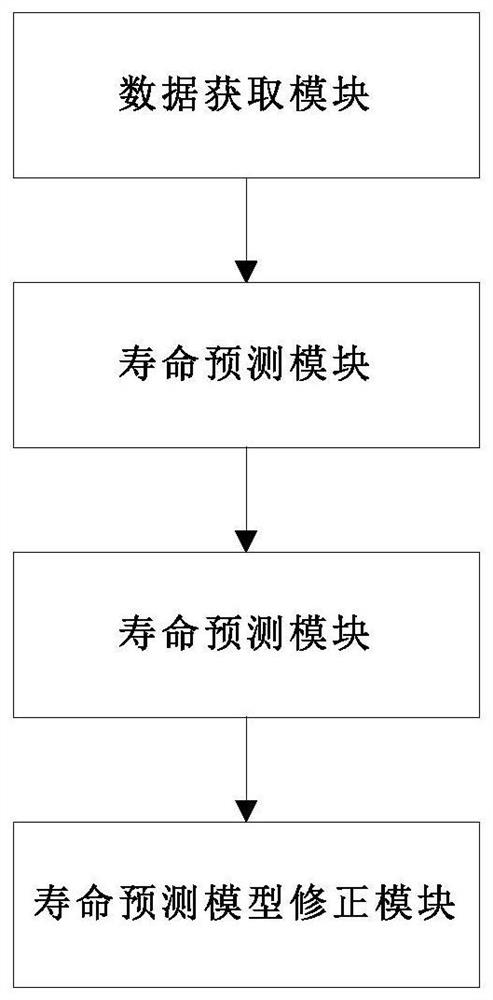
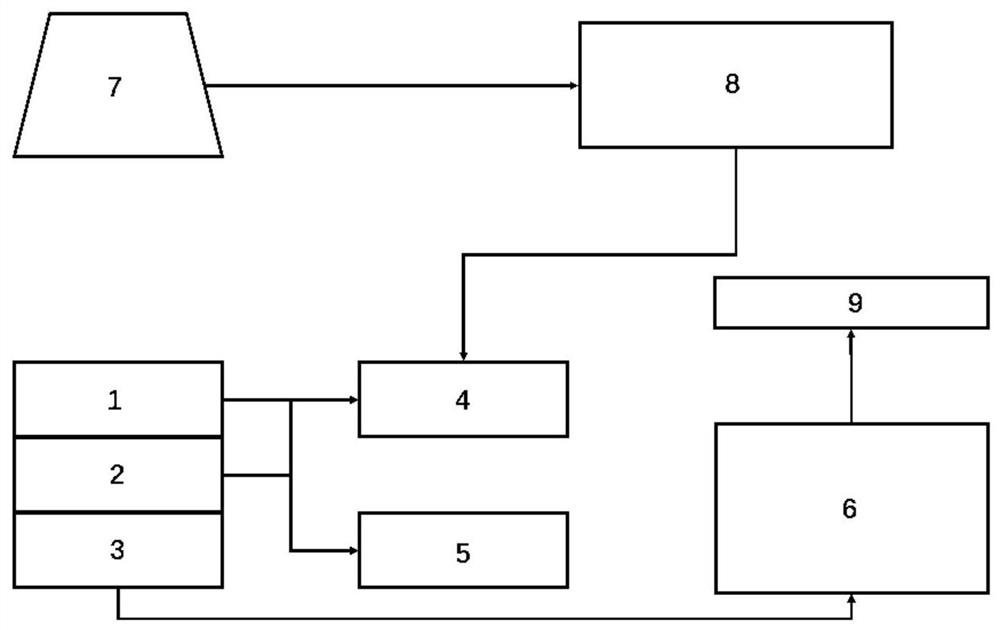
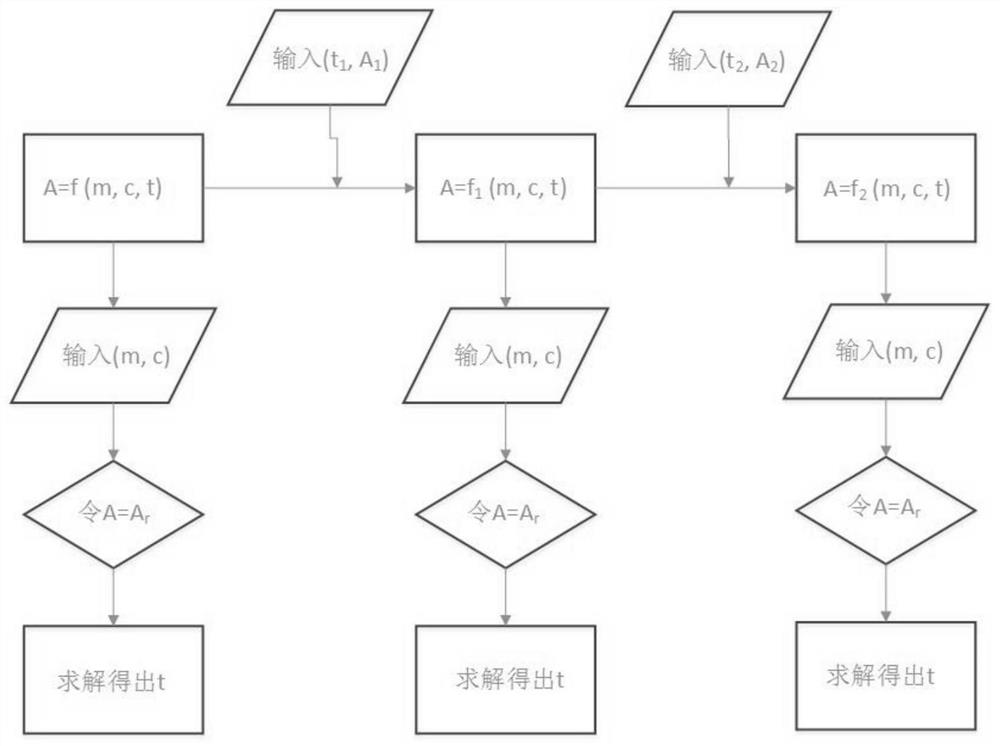
A thermal barrier coating service life measuring and calculating method and device
PendingCN112765904ARapid detection meansAccurate calculationDesign optimisation/simulationPrediction algorithmsCrazing
The invention discloses a thermal barrier coating service life measuring and calculating method. The method comprises the steps of obtaining historical use data of a user, component structure information of a thermal barrier coating and planned use working conditions of components; obtaining a life prediction model of the thermal barrier coating according to the obtained user historical use data, the component structure information of the thermal barrier coating and the planned use condition of the component; measuring a microstructure damage factor of the thermal barrier coating; correcting the fitting coefficient of the life prediction model through the measured microstructure damage factor of the thermal barrier coating, obtaining the corrected life prediction model, and obtaining the life of the thermal barrier coating. A thermal barrier coating nondestructive testing device is adopted to measure crack distribution and TGO layer key parameters of a service part, the measured data can be used for verifying and correcting a thermal barrier coating service life prediction algorithm and CFD boundary conditions, the accuracy of thermal barrier coating service life prediction is improved, and dynamic prediction of the thermal barrier coating service life in the part service period is achieved.
Owner:苏州先机动力科技有限公司
Fluorescent PCR method to identify drug-resistant mutations of Campylobacter jejuni and macrolides
ActiveCN103421892BReduce the cost of trainingGuaranteed accuracyMicrobiological testing/measurementFluorescence/phosphorescenceBacteroidesNucleotide
The invention belongs to the field of bacterial drug-resistant molecular detection, and relates to a multiple real-time fluorescent PCR method for identifying the drug-resistant mutation of macrolides and for identifying Campylobacter jejuni. The method is characterized in that an idiocratic primer, MGB probe, and combination of both are designed, not only is the specificity identification performed for the Campylobacter jejuni, but also the mutation of nucleotide of 2074th and 2075th of the 23S rRNA gene of Campylobacter jejuni and that of nucleotide of 170th and 221st of L4 flagellum gene rp1D of ribosome protein can be detected at the same time. The quick detection for drug-resistant mutation points of the various macrolides and the specificity identify for the Campylobacter jejuni are realized, which is first realized in the same reaction system, so that the identification and drug tolerance detection for the Campylobacter jejuni in clinical trials is greatly reduced, and as a result, a novel method is provided for Campylobacter jejuni drug-resistant monitoring and medicines for clinical trials infection treatment.
Owner:HUAZHONG AGRI UNIV
Application of neuraminic acid and neuraminidase inhibitors in chronic heart failure
ActiveCN109799330BEasy diagnosisGood treatment effectOrganic active ingredientsBiological testingNeuraminic acidPharmaceutical drug
The invention discloses the application of neuraminic acid and neuraminidase inhibitor in chronic heart failure, and belongs to the field of diagnosis, prevention and treatment of cardiovascular diseases. The invention discovers a new biological marker-neuraminic acid, And confirmed the role of neuraminic acid as a diagnostic, prognostic biomarker and risk stratification in chronic heart failure, as well as its role as a drug target in the prevention, remission and / or improvement of chronic heart failure; therefore, the kit was used to detect nerve The amino acid level can be used to predict and assist in the diagnosis of chronic heart failure or evaluate the prognosis of chronic heart failure, and control the level of neuraminic acid through drugs to carry out more intensive diagnosis and follow-up treatment of the disease, obtain a favorable course of disease, and clinically Has huge application value.
Owner:TONGJI HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com