GeXP rapid detection kit for identifying eight types of porcine viral diseases and primer group thereof
A detection kit and a technology for swine virus disease, which are applied in the field of swine virus disease detection, can solve the problems of reagents or kits that have not yet been reported for various infectious disease pathogens, and achieve the resolution of amplification preference, high sensitivity and application prospects. expansive effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment 1, the design of specific primer pair
[0020]Using the primer sequence published by GenBank as a reference, download the known sequence from NCBI and use DNASTAR software for sequence alignment, select highly conserved and specific gene fragments for each target gene, and design 9 specific primers by primer premier5.0 software , and a GeXP universal primer (Table 1) was added to the 5' end of the primer.
[0021] Table 1 Primer Information
[0022]
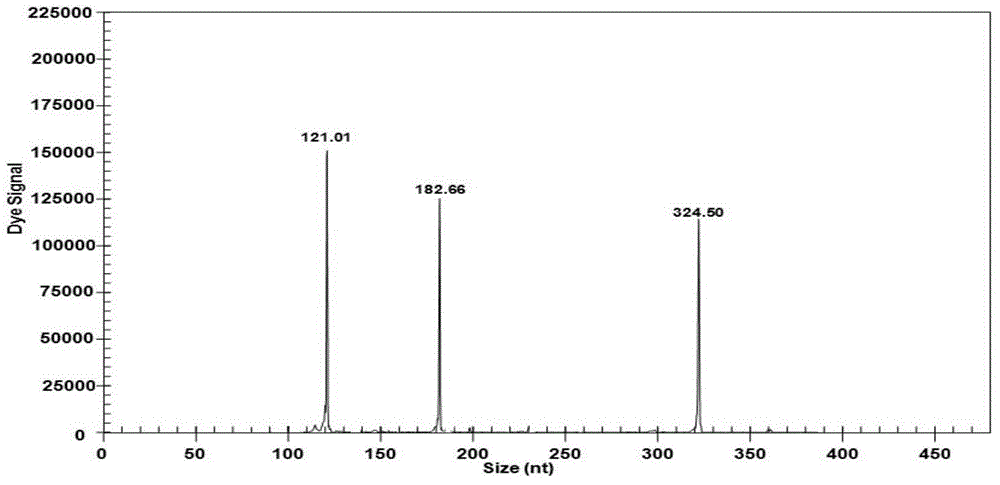
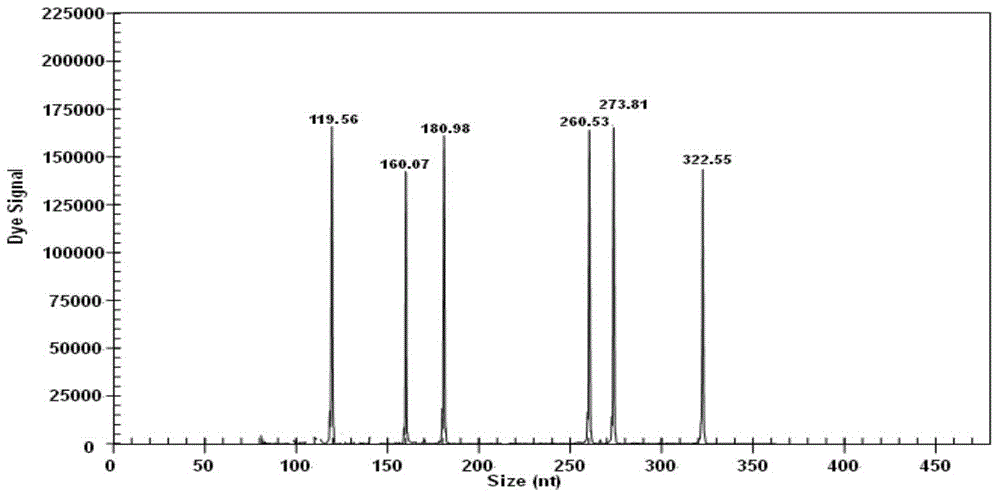
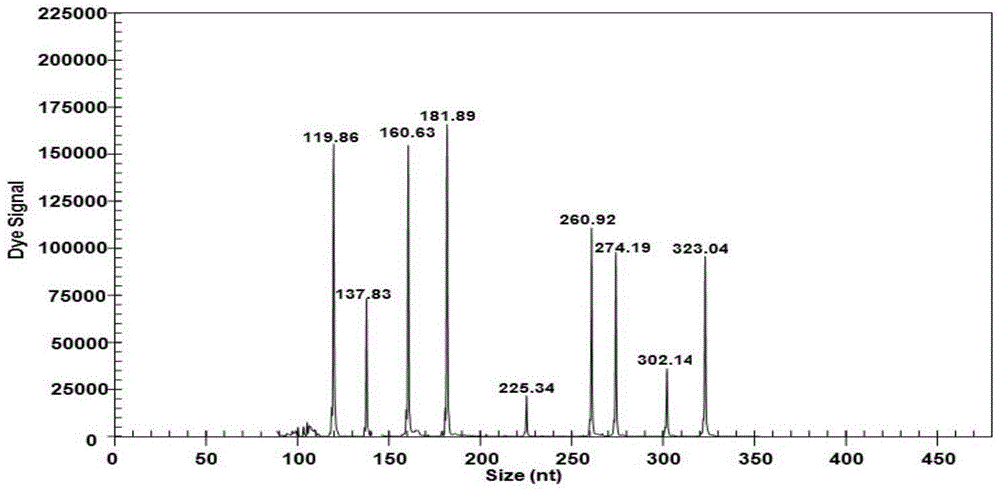
[0023] In Table 1, the codes of the merged bases are R=A / G, D=A / G / T, Y=C / T, and the underline indicates the sequence of the upstream and downstream universal primers; the fluorescent dye Cy5 marks the label of the upstream universal primer, and the downstream primer Not marked. According to the different strains of the pathogens actually detected and the error of the GeXP system (such as the GenomeLabTM GeXP Genetic Analysis System capillary electrophoresis instrument), the length of the actual amplified pr...
Embodiment 2
[0025] Embodiment 2, the specific detection of PCR primer pair
[0026] 1. Template preparation
[0027] 1. Extraction of viral RNA and acquisition of cDNA
[0028] 1) Extraction of viral RNA
[0029] Using DNA / RNA extraction kits (all purchased from Beijing Quanshijin Biotechnology Co., Ltd., catalog number ER201), according to the kit instructions, respectively extract swine influenza virus (H1N1, H1N2, H3N2), porcine Japanese encephalitis virus (JEV ), the RNA of American porcine reproductive and disorder syndrome virus (PRRSV), classical swine fever virus (CSFV)
[0030] 2) Acquisition of cDNA
[0031] The RNA samples obtained in step 1) were reverse-transcribed according to the following reaction system and reaction conditions to obtain cDNA; DEPC water was used as the control of total RNA.
[0032] Reaction system (25 μL): 5×Reverse Transcriptase Buffer 5 μL, 50 pmol Random Primer (9mer) 1 μL, dNTP Mixture (10 mM) 2 μL, 40U Ribonuclease Inhibitor 0.5 μL, 5U / μL AMV Re...
Embodiment 3
[0080] Embodiment 3, the sensitivity detection of PCR primer pair
[0081] 1. Preparation of monoclonal plasmid standards containing target genes
[0082] Swine influenza virus (H1N1, H1N2, H3N2), porcine pseudorabies virus, swine fever virus, American type porcine reproductive and disorder syndrome virus, porcine encephalitis virus, porcine parvovirus, porcine The cDNA or DNA sample of circovirus type 2 is used as a template, and the M gene of swine influenza virus obtained by PCR amplification, the HA gene of H1 subtype swine influenza virus, the HA gene of H3 subtype swine influenza virus, the GD gene of porcine pseudorabies virus, The E2 gene of classical swine fever virus, the NSP2 gene of American type porcine reproductive and disorder syndrome virus, the M protein gene of porcine encephalitis virus, the VP2 gene of porcine parvovirus, and the ORF1 gene of porcine circovirus type 2 were respectively connected with the vector pMD18-T vector ( Purchased from Dalian Bao Bi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com