VIb subtype pigeon Newcastle disease positive serum standard substance and preparation method thereof
A technology for positive serum and standard substance, which can be used in material testing products, measuring devices, instruments, etc., and can solve the problems of low antigen homology, long genetic distance, and low homology.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
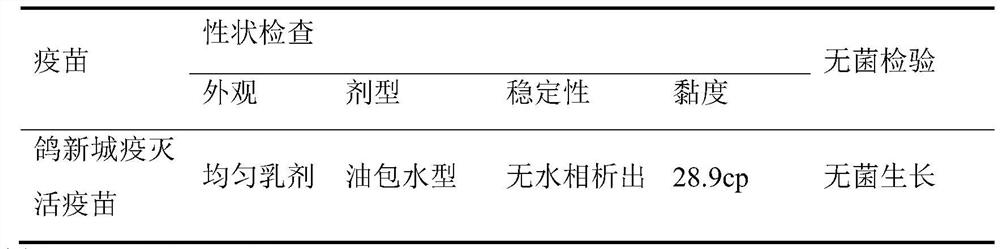
[0041] Embodiment 1——Preparation of Pigeon Newcastle Disease Inactivated Vaccine
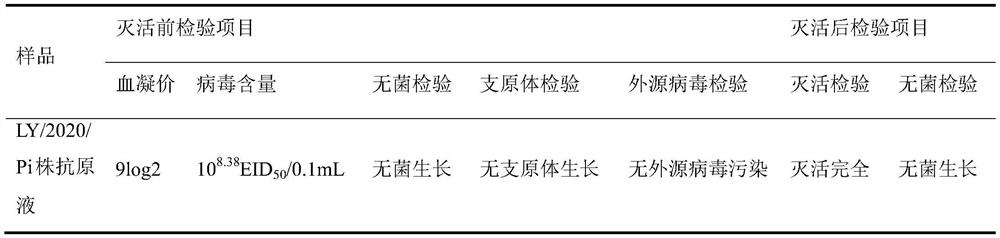
[0042] 1.1 The preparation of the antigen solution uses sterile saline for pigeon Newcastle disease virus LY / 2020 / Pi strain seed 10 -4 Dilute, inoculate 10-day-old SPF embryos into the allantoic cavity, 0.1 mL per embryo, continue incubation at 37°C, discard chicken embryos that died before 48 hours. At 96 hours, the chicken embryos were taken out and frozen, the allantoic fluid was harvested aseptically, and the hemagglutination value and virus content were determined by sampling. At the same time, the sterility, mycoplasma and exogenous virus tests were carried out according to the appendix of the 2015 edition of "Chinese Veterinary Pharmacopoeia". Add β-propiolactone to the qualified embryo fluid at 1 / 4000 (V / V), mix thoroughly, inactivate at 4°C for 20 hours, hydrolyze at 37°C for 2 hours, and store at 2-8°C. At the same time, samples were taken for sterility test and inactivation test. Th...
Embodiment 2
[0051] Embodiment 2 - the screening of negative pigeon
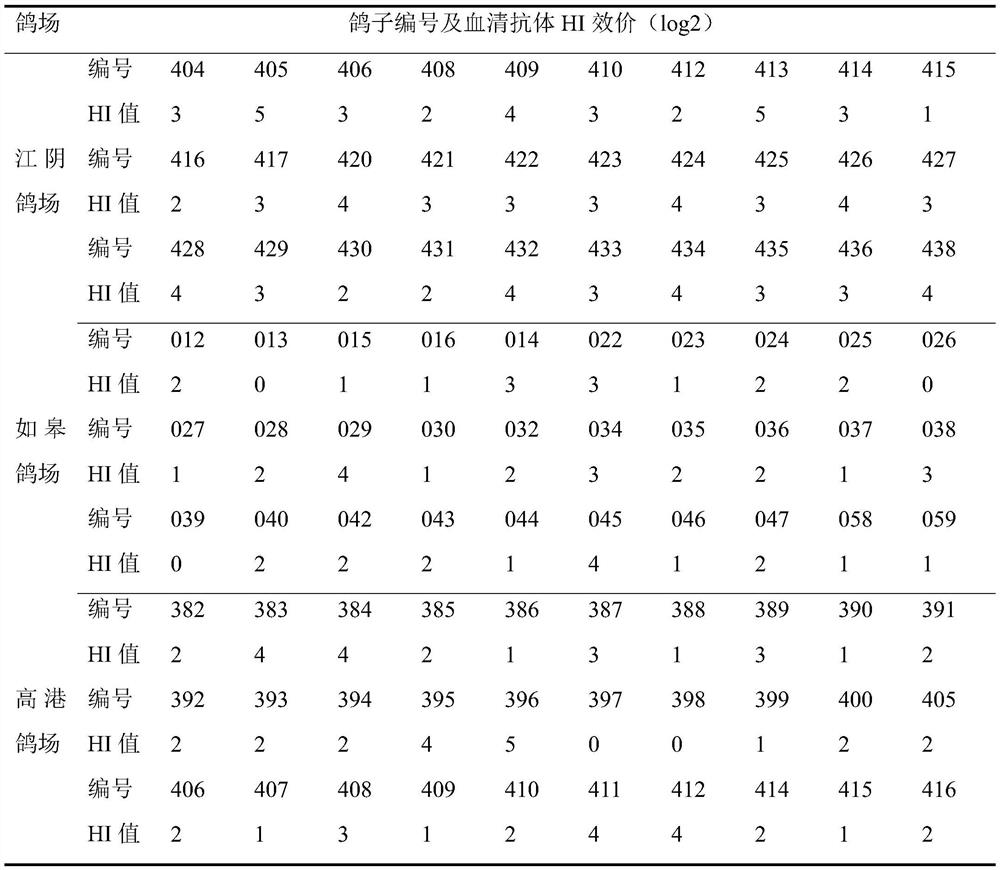
[0052] 1. Primary screening. Select a pigeon farm in Jiangyin, Rugao and Gaogang, Taizhou for investigation, check the breeding and management conditions of the pigeon farm, ask about the history of disease, vaccination history and veterinary drug use of the pigeon farm. Exclude pigeons with poor feeding and management conditions and that cannot meet the following conditions: 1. Normal food intake and drinking water, shiny feathers, strong vigilance, spiral-shaped feces, and a little white urea; 2. No bacteria, viruses, and parasites and other disease history. After investigation, the breeding and management conditions of the pigeon farms in the three places are relatively good, and no bacterial, viral and parasitic infections have occurred within a year. One pigeon farm with normal spirit, food intake, drinking water, feces, growth and development, etc. was selected from each of the three pigeon farms. Pigeon flocks s...
Embodiment 3
[0058] Embodiment 3——Preparation of Pigeon Newcastle Disease Negative Serum
[0059] The pigeons numbered 397 and 398 screened in Example 2 were aseptically collected blood via the subwing vein. After collection, they were placed in a water bath at 37°C for 30 minutes, centrifuged at 5000r / min for 10min, and the serum was collected. After purification, Use a 0.22μm disposable filter to filter and sterilize, store in 1.0mL / bottle aliquots, and use it as negative serum for HI test and sterility, mycoplasma and exogenous virus tests. The results showed that the HI test of the prepared negative serum was negative, and the purity was good, without bacteria, mold, mycoplasma and exogenous virus contamination. The results of the purity test are shown in Table 4.
[0060] Table 4 Pigeon Newcastle disease positive serum and negative serum purity test results
[0061]
[0062] Note: "-" means no microbial growth or negative.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com