In-situ vaccine and preparation method thereof
A vaccine preparation and in-situ technology, which is applied in the fields of pharmaceutical formulations, cancer antigen components, vertebrate antigen components, etc., can solve the problem of unsatisfactory vaccination system, low specific immune response of vaccination response, reduction of vaccine immunogenicity and effective In order to achieve the effect of green preparation method, improve the utilization rate of antigen, improve safety and curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] The present invention provides a method for preparing an in situ vaccine, comprising the following steps:
[0031] S1. Cultivation: resuscitate and culture the tumor cells in the culture medium to reach the logarithmic growth phase, collect the cells with the digestive fluid and wash to obtain the tumor cell fluid;
[0032] S2, inactivation: inactivating the tumor cell liquid collected in step S1, incubating the inactivated tumor cell liquid, centrifuging the incubated tumor cell liquid to take the supernatant to obtain the inactivated antigen stock solution;
[0033]S3. Preparation of nanomaterial @Mal solution: use nanomaterial and PEG-Mal ligand in a mass ratio of 1:10-1:15 to form a mixed solution, add 10-15 times the volume of the mixed solution of cyclohexane to mix Precipitate the liquid, remove the supernatant by centrifugation to obtain the precipitate, which is dried, dissolved in ultrapure water, centrifuged and passed through a membrane, and then ultrafilter...
Embodiment 1
[0044] Prepare an in situ vaccine as follows:
[0045] (a) The breast cancer tumor cells (4T1) were recovered and cultured to reach their logarithmic growth phase. After the 4T1 cells were observed under the microscope and covered the bottom of the culture dish, the unattached tumor cells were washed with PBS buffer, and then used with a mass fraction of The adherent tumor cells were brought into suspension by digestion with 0.2% trypsin solution, collected by centrifugation, and washed 4 times with PBS buffer to remove the complete medium components in the culture dish, so that the tumor cells were suspended in PBS buffer The tumor cell fluid was formed, and then counted, and the adjusted cell volume was about 4M;
[0046] (b) irradiating the collected cells with X-rays, then incubating in a cell incubator for 0.5 h, then centrifuging to remove cells and cell debris, collecting the supernatant and centrifuging again to remove residual cell debris, for later use;
[0047] (c)...
Embodiment 2
[0051] Prepare an in situ vaccine as follows:
[0052] (a) with embodiment one;
[0053] (b) with embodiment one;
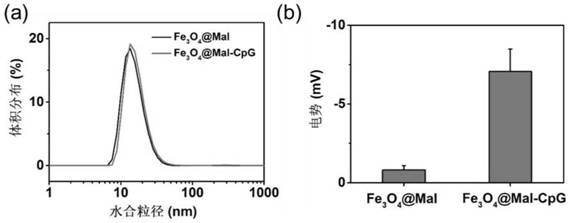
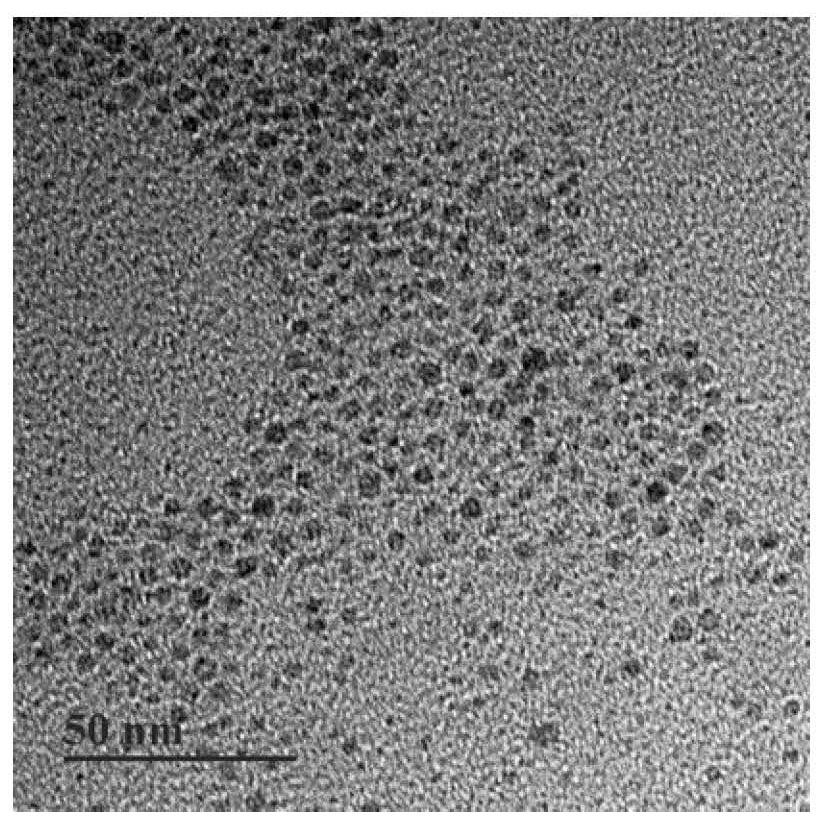
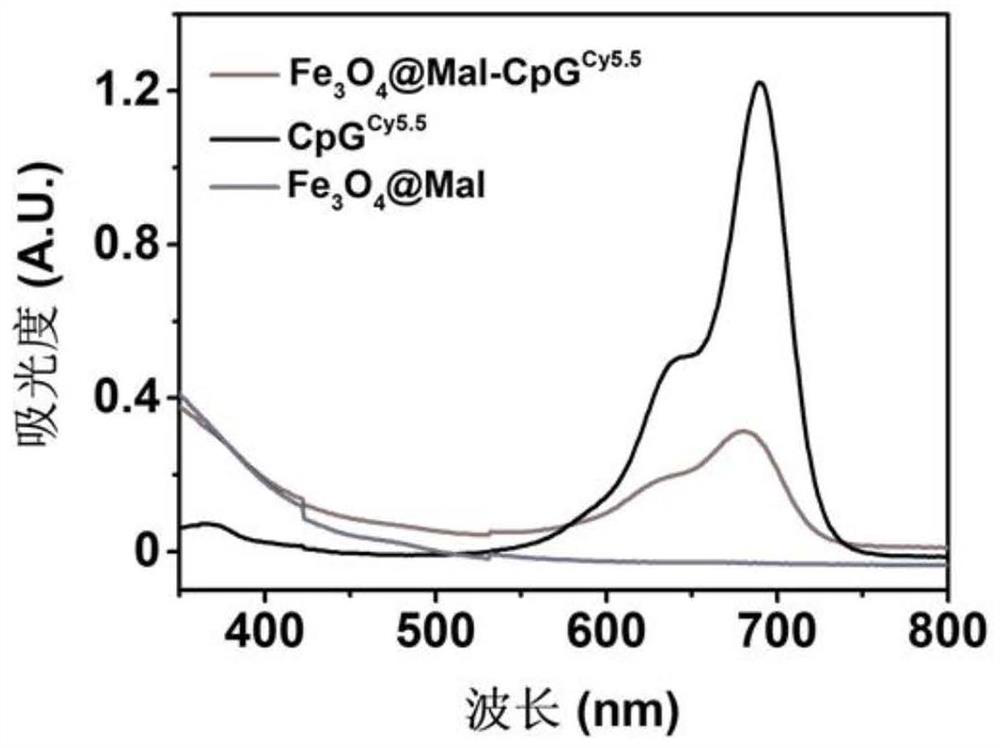
[0054] (c) First, draw 2 mL of oil-phase Fe with a concentration of 6.6 mg / mL 3 O 4 into a 15mL centrifuge tube, the oil phase Fe 3 O 4 Contain cyclohexane, add 15 mL of acetone solution to the centrifuge tube to complete precipitation, then centrifuge at 5000g for 5 min to remove the supernatant, and then add 2 mL of tetrahydrofuran solution until the precipitation is completely dissolved. Then weigh 158 mg of PEG-Mal ligand, at this time the oil phase Fe 3 O 4 The mass ratio of PEG-Mal ligand is =1:12, and tetrahydrofuran solution is added dropwise to it and shaken to dissolve it completely. Fe dissolved in tetrahydrofuran 3 O 4 Added to PEG-Mal also dissolved in tetrahydrofuran, and sonicated for 1 h. Cool to room temperature, then add 10 times the volume of cyclohexane, remove the supernatant by centrifugation, add an appropriate amount of ultrapure...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com