Immunologic adjuvant composition as well as preparation method and application thereof
A vaccine composition and composition technology, applied in biochemical equipment and methods, vaccines, microorganisms, etc., to achieve good stability, simple preparation process, and remarkable immune effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
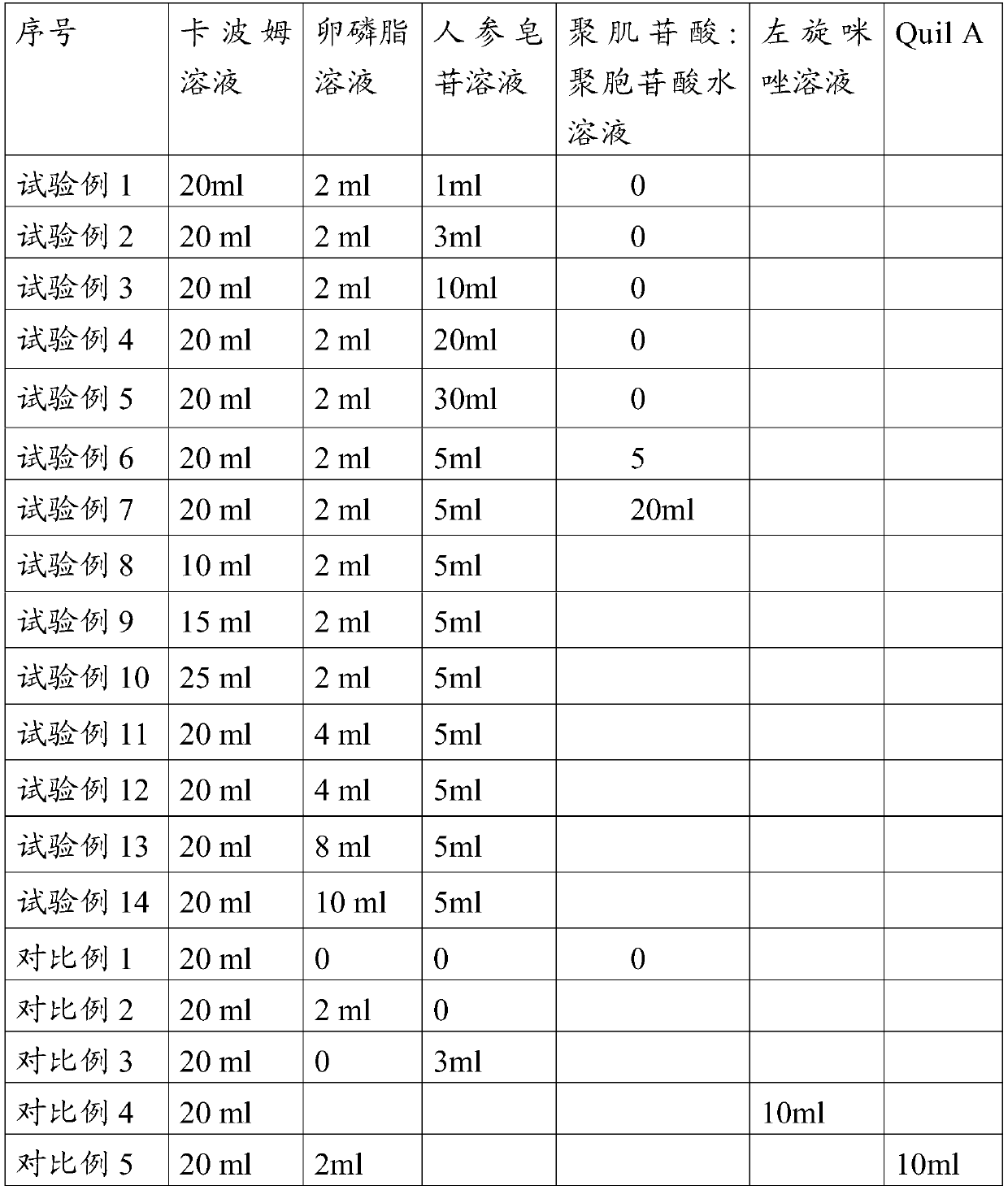
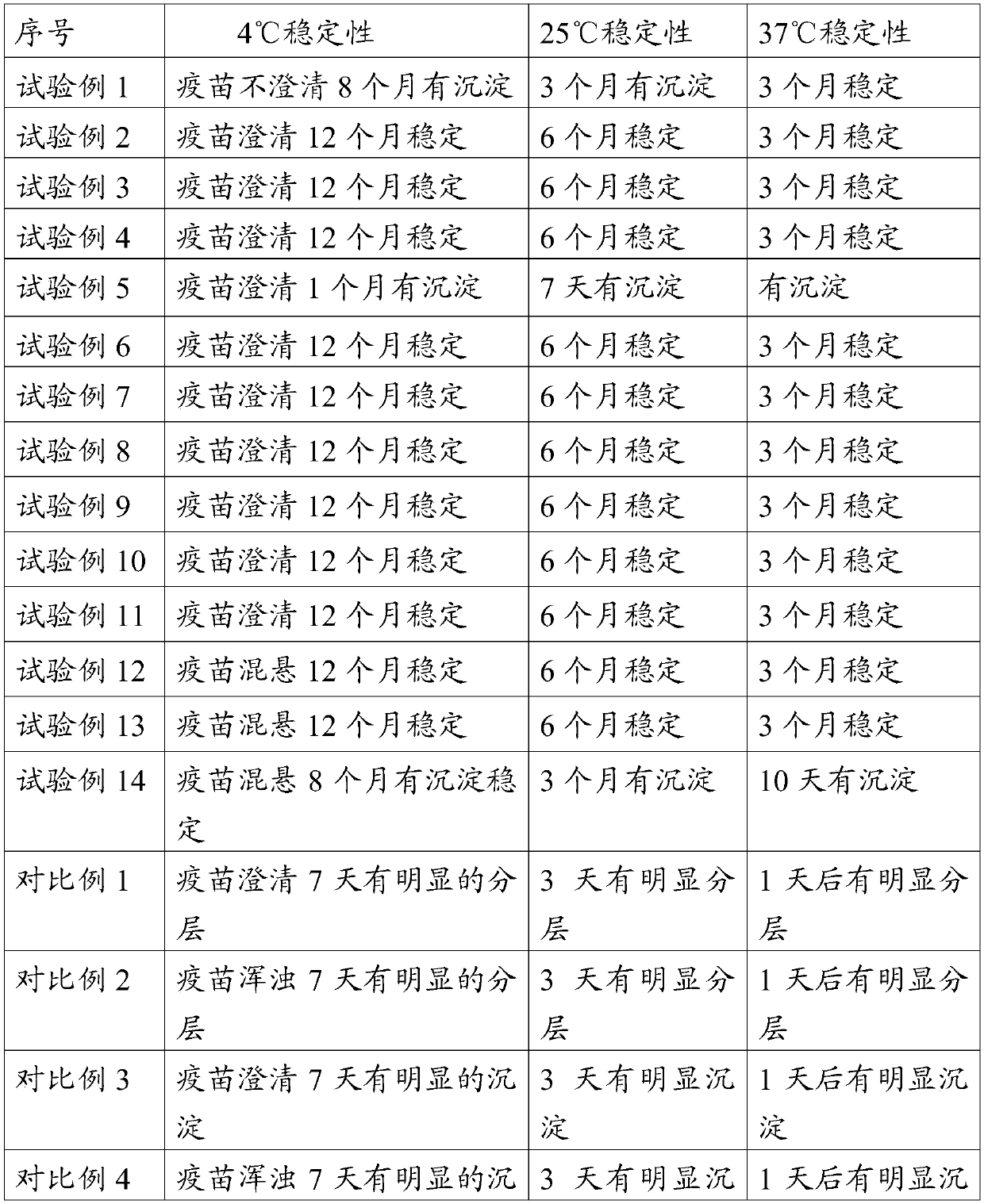
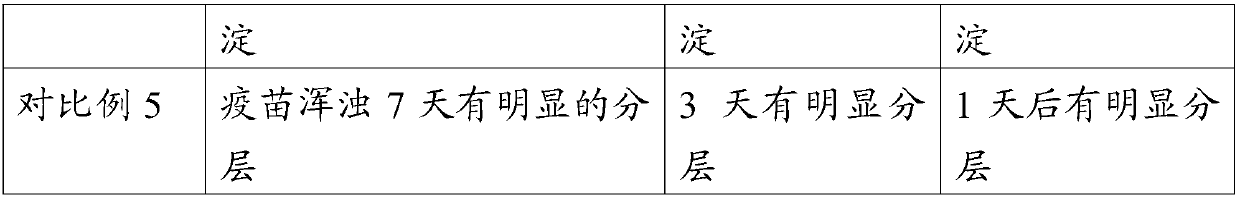
Embodiment 1
[0057] Embodiment 1 prepares carbomer solution
[0058] Add 1 g of Carbomer 934 to 50 ml of water, stir to dissolve, then add 1.5 g of sodium chloride solution, adjust the pH to 7.0 with sodium hydroxide solution, and sterilize it by autoclaving for later use, with a content of 2% w / v.
Embodiment 2
[0059] Embodiment 2 prepares lecithin ethanol solution
[0060] Add 5 g of lecithin (PC90, produced by Jiangsu Manshi Biotechnology Co., Ltd.) into 100 ml of ethanol, stir to dissolve it, and filter it through a 0.22-micron filter membrane for later use, with a content of 5% w / v.
Embodiment 3
[0061] Embodiment 3 prepares ginsenoside solution
[0062] Add 1 g of ginseng stem and leaf saponins (containing 76.7% of total saponins, produced by Jilin Hongjiu Biotechnology Co., Ltd.) into 100 ml of water, stir to dissolve, filter with a 0.22 micron membrane filter, and set aside, with a content of 1% w / v.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com