Newcastle disease and H9 subtype avian influenza bivalent inactivated vaccine containing immunopotentiator as well as preparation method and application of newcastle disease and H9 subtype avian influenza bivalent inactivated vaccine
A dual inactivated vaccine and immune enhancer technology, applied in the field of immune adjuvant technology and biopharmaceuticals, can solve the problems of shortening the protection blank period and slow generation of immune protection, achieving long-lasting immune protection effect and improving bioavailability the effect of reducing toxicity and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
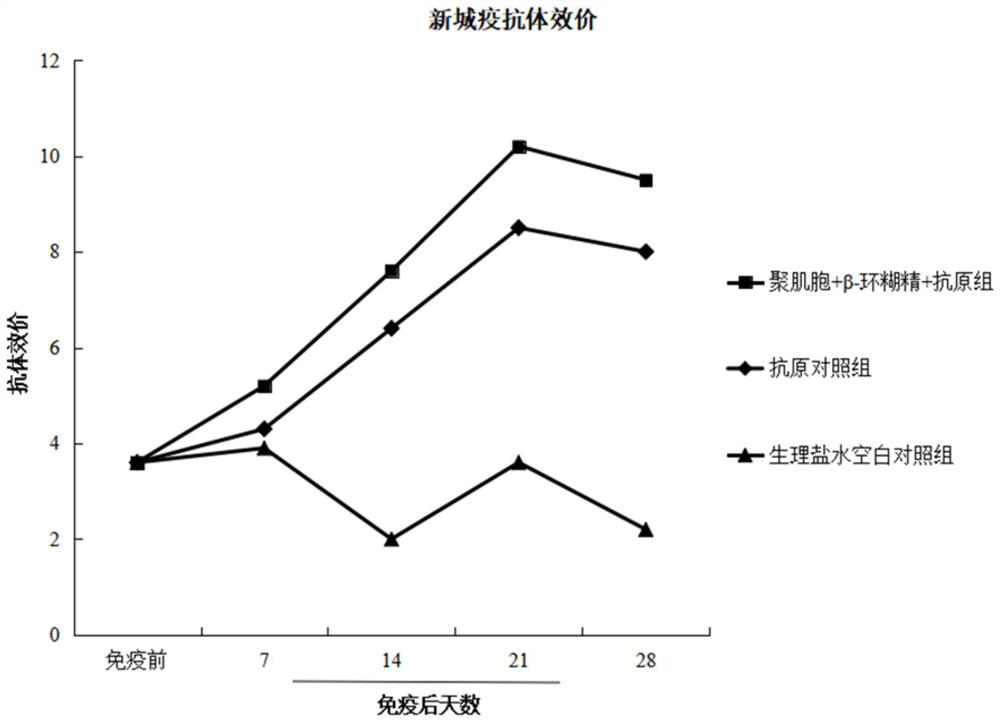
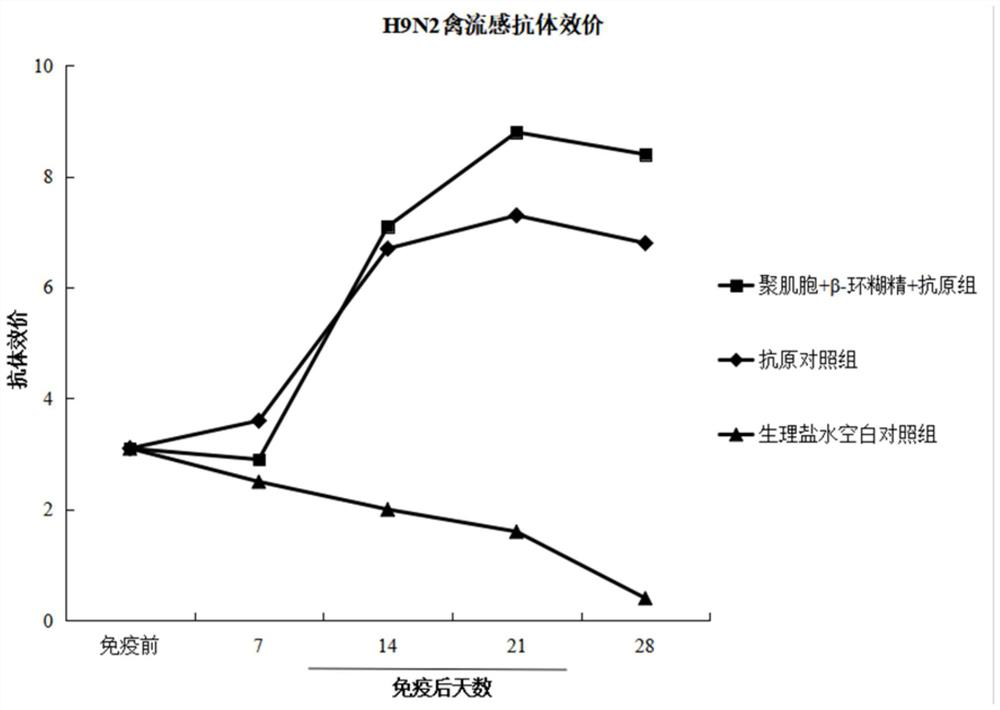
Image
Examples
example 1
[0077] 1. Experimental materials
[0078] The inactivated Newcastle disease virus liquid and the inactivated avian influenza H9N2 subtype virus liquid are inactivated by 1% formaldehyde solution.
[0079] 14-day-old healthy white-feathered broiler chickens.
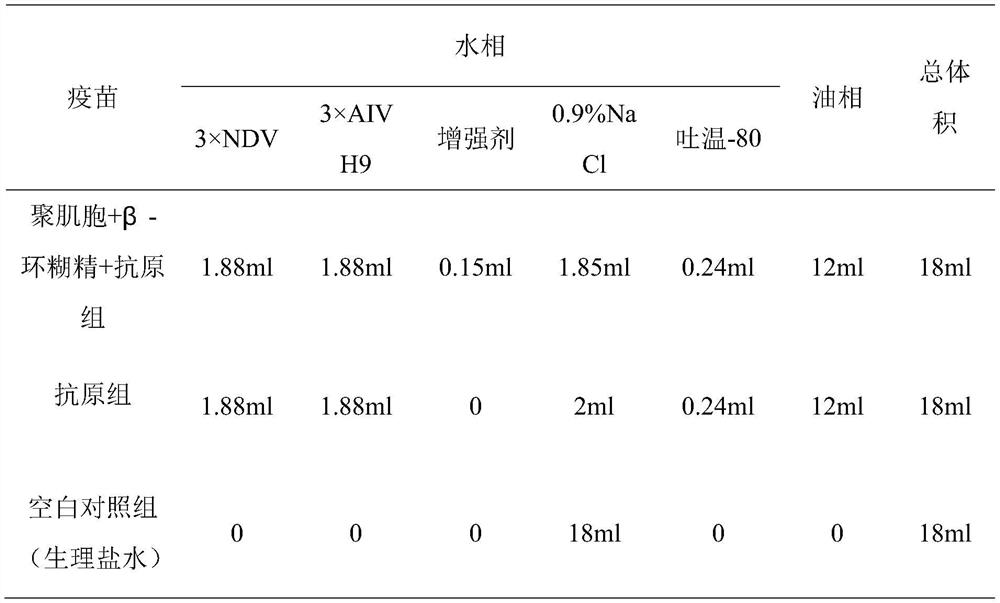
[0080] 2. Preparation of the vaccine
[0081] Weigh 10 mg of polymyocytes, dissolve them in 1 ml of normal saline, and make No. No. 2 mother liquor of 20mg / ml. All filter sterilized.
[0082] The immune enhancer, inactivated Newcastle disease virus liquid, inactivated avian influenza H9N2 subtype virus liquid and Tween-80 (Tween 80) were prepared according to the volume ratio of 2: 1.88: 1.8: 0.24 water phase solution, added Tween Immediately after that, put in a water bath for about 5 minutes, shake to mix well. The volume ratio of Span-80 and white oil was 1:19, and 0.06 mg of aluminum stearate was added to prepare the oil phase. Stir for 5 minutes by a high-speed emulsifier at 13000 rpm / min to obtain experimental...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com