Methods and compositions for reconstitution of small glial cells
A cell, homozygous technology, applied in nervous system cells, other methods of inserting foreign genetic materials, drug combinations, etc., can solve the problems of fast and slow progress of nervous system diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
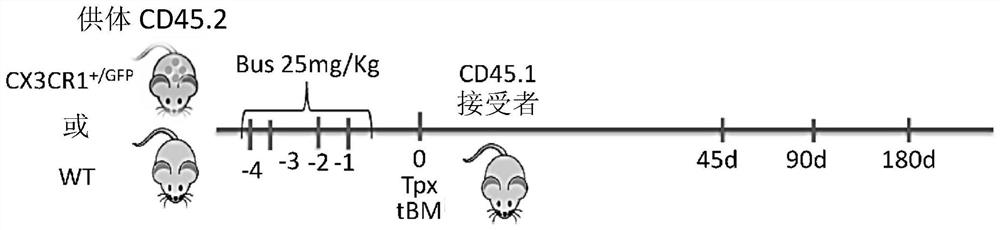
[0133] Example 1: CX3CR1 + / GFP Transplantation of hematopoietic cells leads to rapid microglial remodeling
[0134] From 8-week-old CD45.2 wild-type or CD45.2 CX3CR1 + / GFPIsolation of bone marrow cells from mice. After red blood cells were lysed, 2.0x10 6 Four cells were transplanted intravenously (IV) into eight-week-old CD45.1 wild-type recipients pretreated with busulfan (25 mg / kg for four days). Transplanted mice were clinically monitored and sacrificed 45, 90 and 180 days post-transplantation ( Figure 1A ). No deaths occurred. Upon sacrifice following perfusion, bone marrow and brain were harvested to monitor engraftment of transplanted cells by flow cytometry. Brain cells were isolated after papain digestion and Percoll density gradient Fenix.
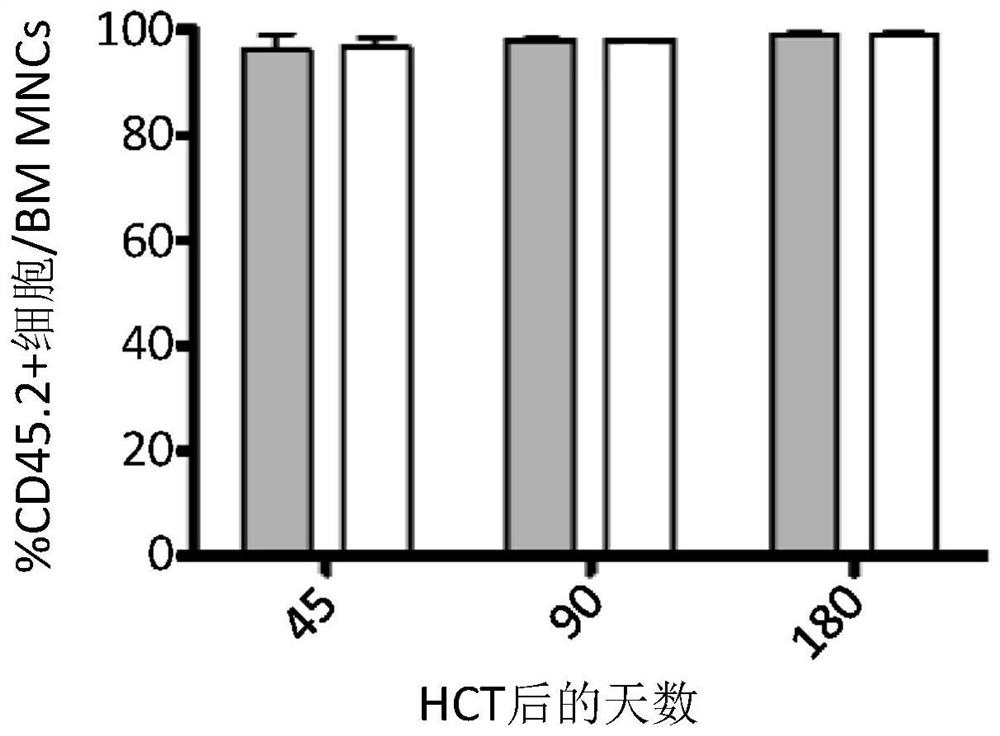
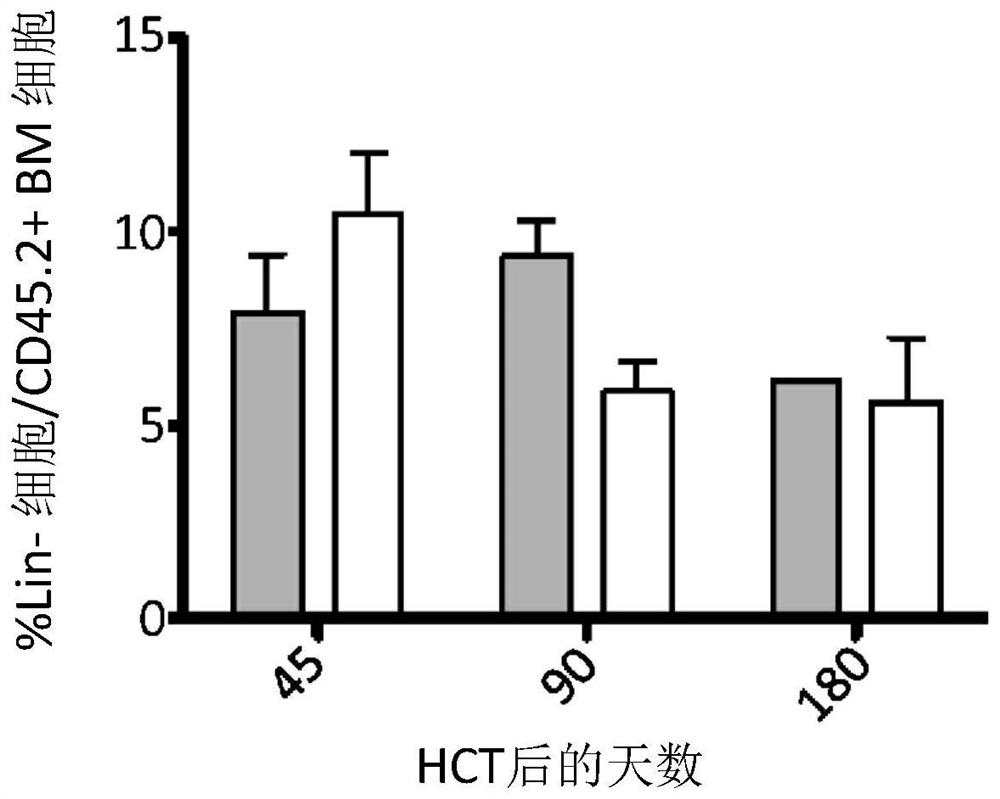
[0135] Transplanted CX3CR1 was observed in recipient bone marrow + / GFP Similar sustained engraftment of cells and wild-type cells ( Figure 1B ). Compared with mice receiving wild-type cells, in mice receiving CX3CR1 ...
Embodiment 2
[0137] Example 2: CX3CR1 + / GFP Hematopoietic stem progenitor cells (HSPCs) have a competitive advantage after transplantation
[0138] Better Characterization of CX3CR1 + / GFP To phenotype the cells and assess whether they had a qualitative (and quantitative) advantage over wild-type, competitive transplantation experiments were performed. Eight-week-old CD45.2 wild-type or CD45.2 CX3CR1 + / GFP HSPCs were isolated from mice, labeled with lentiviral vectors encoding different fluorescent markers (PGK.mCherry and PGK blue fluorescent protein (BFP), respectively), and co-transplanted with CD45.1busulfan at a 1:1 ratio Among bone marrow recipients ( Figure 3A ).
[0139] Transplantation was performed in separate experiments using intravenous (IV) and intracerebroventricular (ICV) administration. In an IV setting, transplant a total of 1.0x10 per mouse 6 HSPC. In the ICV environment, a total of 0.3x10 per mouse transplantation 6 HSPC. Mice receiving HSPCs administered by IC...
Embodiment 3
[0143] Example 3: CX3CR1 + / GFP HSPCs generate more mature microglia-like cells than wild-type HSPCs after transplantation
[0144] To demonstrate that CX3CR1 haploinsufficiency might also be associated with faster maturation of suppressed cells and / or their progeny, particularly in the brain, the morphology and Expression of microglia-associated genes.
[0145] Different parameters reported in the literature, which are valuable criteria for describing microglial morphology (Verdonk et al. 2016), were evaluated. These parameters include the total length of the branches (Sum length); the complexity index (CI), defined as the ratio of the number of segments per cell to the number of primary branches, where segments are the length of the process between two base points; coverage The area of surroundings (COA), which defines the total 2D surface covered by a cell's branches and is described as the area of the polygon formed by connecting the ends of its processes, in μm 2 e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com