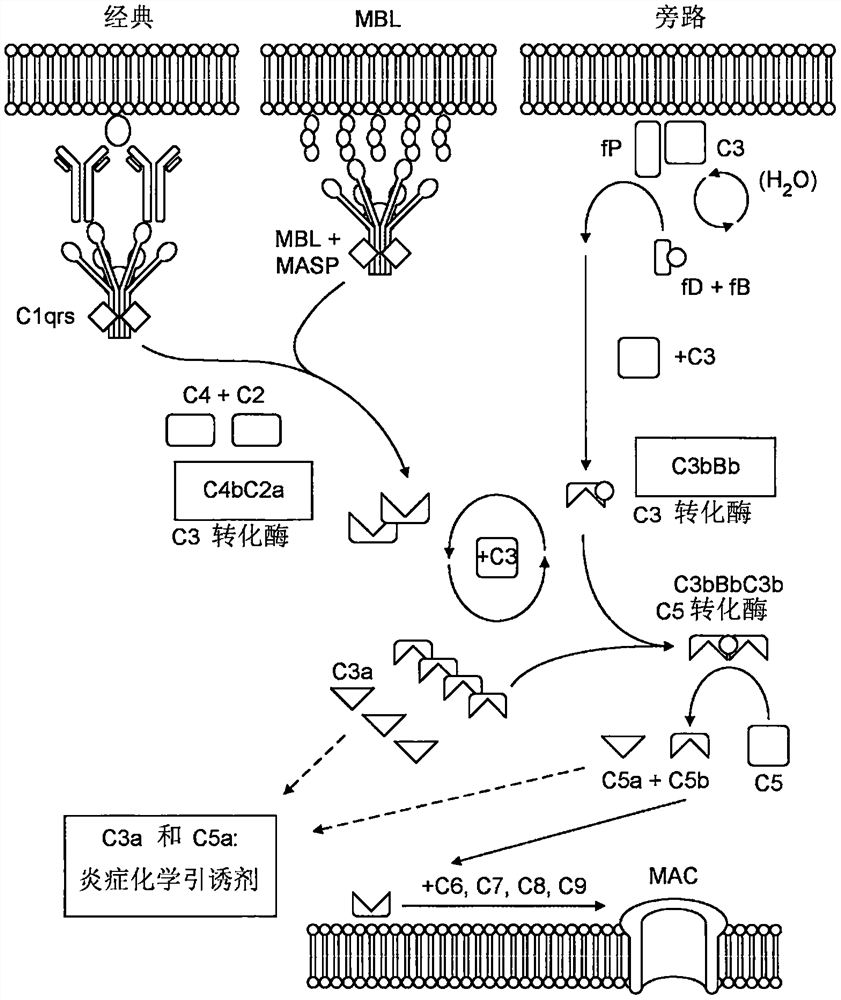
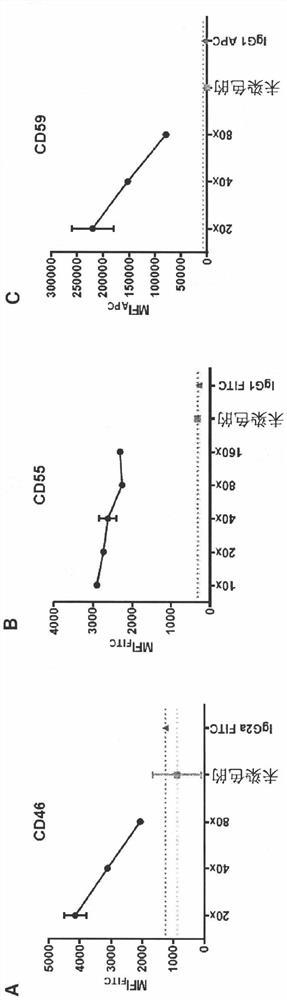
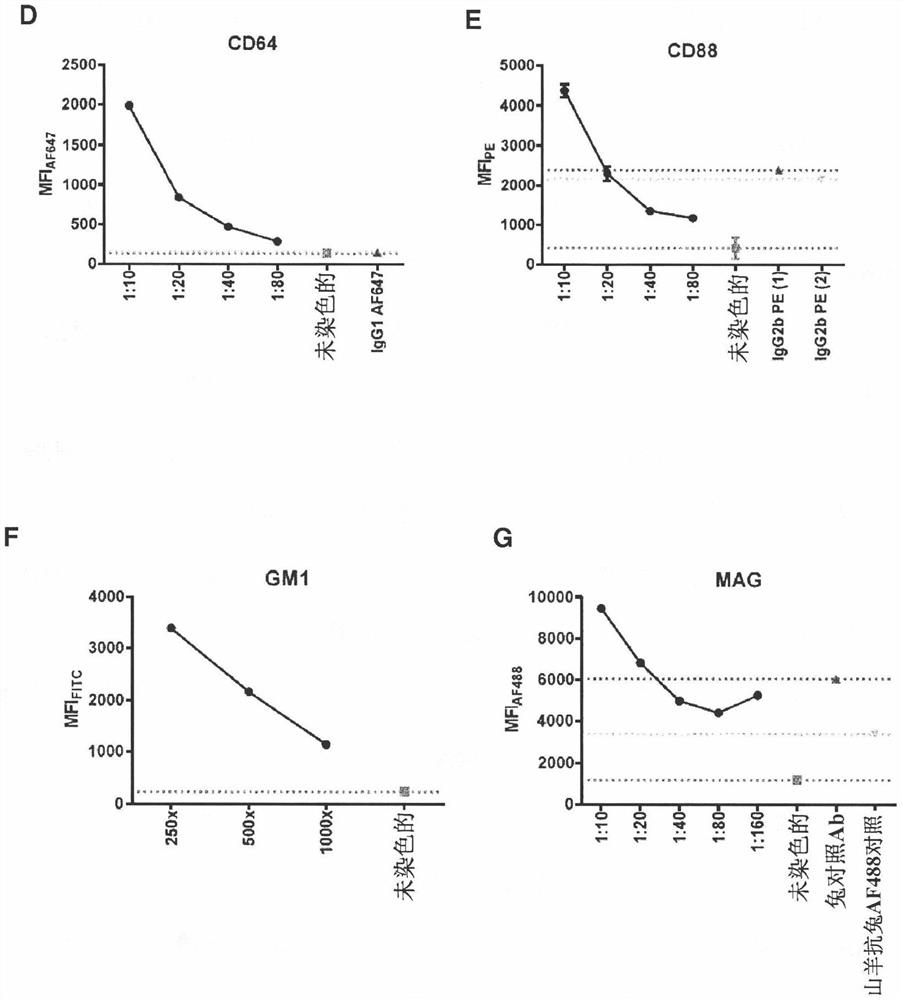
Antagonists of complement system for use in methods of treating paraproteinemia neuropathy
A complement system, neurological technology, applied in neurological diseases, blood diseases, immunoglobulins, etc., can solve problems such as the complexity of the complement cascade
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0216] Example 1 Complement Inhibition Using Live Schwann Cells in an In Vitro Model of Multifocal Motor Neuropathy (MMN)
[0217] Schwann cells are myelin-secreting glial cells that spirally wrap around axons in the peripheral nervous system to form myelin sheaths. These cells attach to axons via the protein GM1. Its most important function is the myelination of axons to improve neuronal jump conduction, but it also contributes to neuronal survival and signaling. Dysfunction of these cells leads to demyelination, which leads to reduced signal transduction. Thus, Schwann cells are associated with several demyelinating disorders such as MMN. There exists a human Schwann cell line - sNF02.2 - and it was derived from lung metastases in patients diagnosed with malignant peripheral nerve sheath tumors. These cells were established from multiple passages of primary tumor material in culture until they formed a homogeneous Schwann-like population that displayed clonal morphology...
Embodiment 2
[0260] Example 2 Complement Inhibition in an In Vitro Model of Multifocal Motor Neuropathy (MMN) Using Fixed Schwann Cells system
[0261] A. method
[0262] 2.1 Protocol for culturing and fixing Schwann cells on coverslips
[0263] Incubate Schwann cells in DMEM medium containing 100 U / mL penicillin, 100 μg / mL streptomycin and supplemented with 10% FCS at 37 °C and 5% CO 2 under cultivation. Twice weekly, cells were passaged or used for experiments when >80% confluency was reached. Discard the medium and wash the cells with 10 ml PBS. To dissociate cells, add 3 mL (T75) or 5 mL (T175) of Accutase cell detachment solution and incubate cells at 37°C for 5 min, or until cells are completely detached. Then, medium was added (7 mL to T75, 10 mL to T175) and cells were transferred to 15 mL tubes, followed by centrifugation (125 x g, 10 min). The pellet was resuspended in 5 ml of medium and counted using trypan blue to differentiate live from dead cells. Next, cells we...
Embodiment 3
[0297] Example 3 Using induced pluripotent stem cells (induced pluripotent stem cells, iPSCs) in multifocal Complement Inhibition in an In Vitro Model of Motor Neuropathy (MMN)
[0298] A. method
[0299] 3.1 Protocol for Spinal Motor Neuron Differentiation from iPSCs
[0300] Motor neuron-like cells (MN) from induced pluripotent stem cells (iPSCs) were prepared as described in the literature (Harschnitz Oet al. J Clin Immunol. 2014, Jul; 34 Suppl 1: S112-9), and with Dr L van Prepared in collaboration with der Pol and colleagues (Department of Neurology, UMCU, The Netherlands). Briefly, human fibroblasts were obtained from skin biopsies of healthy individuals according to an institutional review board-approved protocol. These cells were cultured in mouse embryonic fibroblast (MEF) medium containing DMEM GlutaMAX supplemented with 10% fetal bovine serum and 1% penicillin / streptomycin at 37°C in 5% CO 2 under cultivation. Cells were reprogrammed within the first 5 p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com