Preparation method and application of icotinib intermediate
A technology of icotinib and intermediates, which is applied in the field of chemical synthesis and can solve the problems of high environmental pollution, low yield of target product, large pollutant discharge and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] A preparation method of an icotinib intermediate, comprising the following steps;
[0036] (1) Add triethylene glycol to the alkaline mixed solution, the alkaline mixed solution is added with four
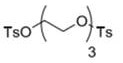
[0037] The mass concentration of hydrofuran is 32% sodium hydroxide solution, and the mixed solution of p-toluenesulfonyl chloride and tetrahydrofuran is added dropwise to carry out esterification reaction. After the reaction is completed, cool, filter, and dry to obtain product 1, the structural formula of product 1 as follows;
[0038]
[0039] (2) Mix the product 1, 3,4 dihydroxybenzonitrile and add dropwise to the mixture of acetonitrile and potassium carbonate
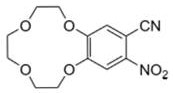
[0040] In the combined solution, carry out cyclization reaction, cool after reaction, filter, dry, obtain product 2, the structural formula of product 2 is as follows;
[0041]
[0042] The reaction equation in this step is as follows;
[0043] The key to this step is ring closure, which has formed ...
Embodiment 2
[0058] A preparation method of an icotinib intermediate, comprising the following steps;
[0059] (1) Add triethylene glycol to the alkaline mixed solution, the alkaline mixed solution is added with four
[0060] The mass concentration of hydrofuran is 33% sodium hydroxide solution, and the mixed solution of p-toluenesulfonyl chloride and tetrahydrofuran is added dropwise to carry out esterification reaction. After the reaction is completed, it is cooled, filtered, and dried to obtain product 1, the structural formula of product 1 as follows;
[0061]
[0062] (2) Mix the product 1, 3,4 dihydroxybenzonitrile and add dropwise to the mixture of acetonitrile and potassium carbonate
[0063] In the combined solution, carry out cyclization reaction, cool after reaction, filter, dry, obtain product 2, the structural formula of product 2 is as follows;
[0064]
[0065] The reaction equation in this step is as follows;
[0066] The key to this step is ring closure, which...
Embodiment 3
[0082] A preparation method of an icotinib intermediate, comprising the following steps;
[0083] (1) Add triethylene glycol to the alkaline mixed solution, the alkaline mixed solution is added with four
[0084] The mass concentration of hydrofuran is 35% sodium hydroxide solution, and the mixed solution of p-toluenesulfonyl chloride and tetrahydrofuran is added dropwise to carry out esterification reaction. After the reaction is completed, cool, filter, and dry to obtain product 1, the structural formula of product 1 as follows;
[0085]
[0086] (2) Mix the product 1, 3,4 dihydroxybenzonitrile and add dropwise to the mixture of acetonitrile and potassium carbonate
[0087] In the combined solution, carry out cyclization reaction, cool after reaction, filter, dry, obtain product 2, the structural formula of product 2 is as follows;
[0088]
[0089] The reaction equation in this step is as follows;
[0090] The key to this step is ring closure, which has formed ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap