Nanosheet-structured NiFeCr composite hydroxide oxygen evolution material prepared by chemical oxidation method
A technology of composite hydroxide and nano-flakes, which is applied in the direction of electrolysis process, electrolysis components, electrodes, etc., can solve the problem that the electrochemical treatment method cannot be used in large-scale and large-scale, so as to save electrolysis energy consumption and avoid easy Shedding problem, avoid practical effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] (1) Cut the stainless steel fiber felt into a shape of 1cm × 1cm, ultrasonically remove the surface oil stains in absolute ethanol for 10 minutes, and then put it in 1mol L -1 The surface oxides were removed by sonication in HCl for 5 min, and finally washed with deionized water and vacuum dried for use.
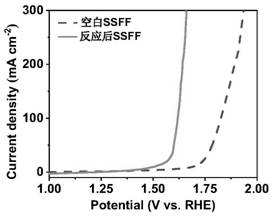
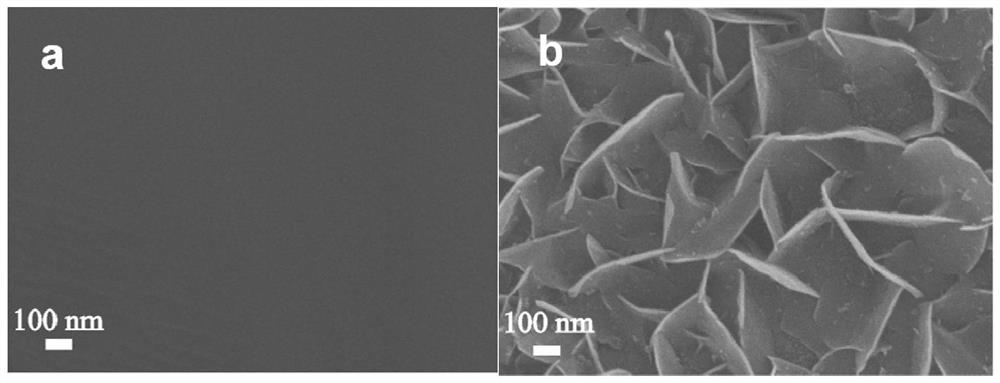
[0018] (2) 0.1mol L -1 of ammonium persulfate and 5mol L -1 The mixed solution of sodium hydroxide was used as the reaction solution, the treated stainless steel fiber felt was placed in the above reaction solution, reacted at 80 °C for 45 min, the stainless steel fiber felt was taken out, washed repeatedly with deionized water, and vacuum dried to obtain a nano-sheet structure. NiFeCr composite hydroxide oxygen evolution material. The obtained electrode material is in 0.5mol L -1 Na 2 CO 3 / NaHCO 3 showed good oxygen evolution catalytic performance in 10mAcm -2 and 100mA cm -2 Only 273mV and 397mV overpotentials are required at the current density of .
Embodiment 2
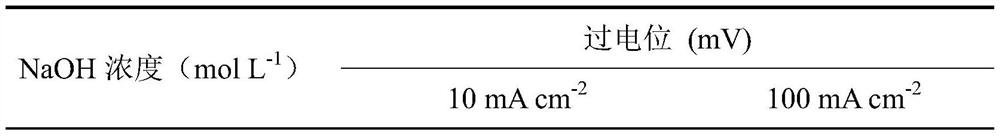
[0020] Referring to the electrode preparation method of Example 1, metal oxide / hydroxide electrode materials were prepared at different concentrations of sodium hydroxide. The concentrations of sodium hydroxide were 1, 3, 5, and 7 mol L -1 , the rest of the conditions are the same as the implementation case 1. The prepared electrode material was taken out, rinsed repeatedly with deionized water, and then vacuum-dried. The obtained electrode material was 0.5 mol L. -1 Na 2 CO 3 / NaHCO 3 The catalytic performance of oxygen evolution is shown in Table 1.
[0021] Table 1 Catalytic performance for oxygen evolution of stainless steel fiber felt electrodes treated with different concentrations of sodium hydroxide
[0022]
[0023]
Embodiment 3
[0025] Referring to the electrode preparation method of Example 1, the metal oxide / hydroxide electrode material was prepared by changing the concentration of ammonium persulfate. The concentration of ammonium persulfate is 0.05mol L -1 , the rest of the conditions are the same as the implementation case 1. The prepared electrode material was taken out, rinsed repeatedly with deionized water, and dried in vacuum. The obtained electrode material is in 0.5mol L -1 Na 2 CO 3 / NaHCO 3 demonstrated good oxygen evolution catalytic performance at 10 mA cm -2 and 100mA cm -2 Overpotentials of 362 mV and 495 mV are required at the current density of .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com