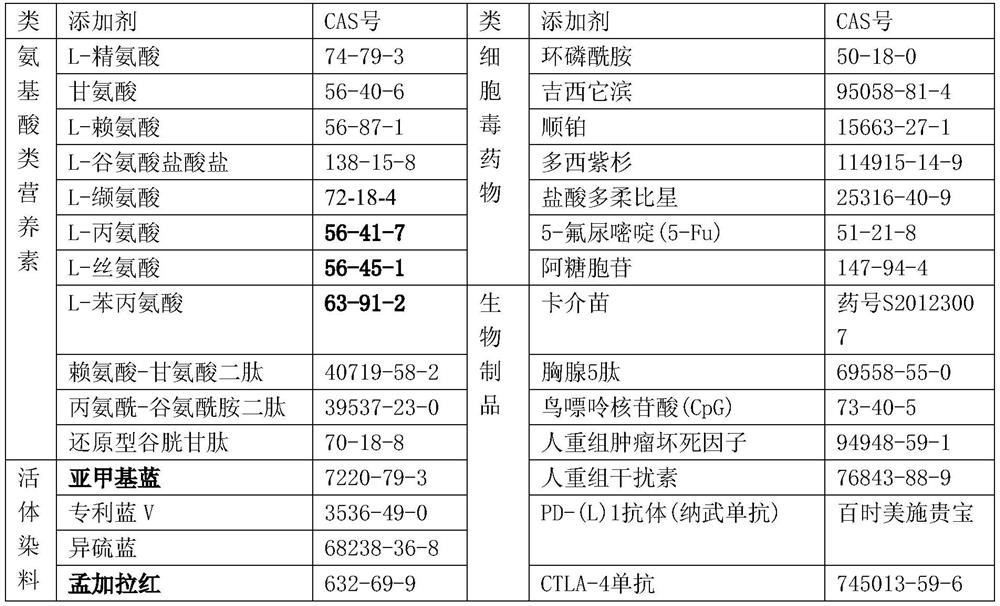
Application of non-pathogenic cell-related component and pharmaceutical composition containing non-pathogenic cell-related component
A non-pathogenic and compositional technology, applied in drug combinations, medical preparations containing active ingredients, drug delivery, etc., to achieve the effects of reducing safety risks, non-toxic systemic safety, and low local irritation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0095] According to the preparation method of the present invention, the preparation of the pharmaceutical composition of the present invention comprises the following steps: preparing an optional drug containing the cell-related components, the synergistic components and other substances that may exist according to the amount ratio and / or the administration concentration. A pharmaceutical preparation for topical administration. In one embodiment, the pharmaceutical composition of the present invention can be prepared by a method comprising the following steps: 1), preparing the cell-related components; 2), administering the required amount according to the concentration required for the therapeutic effect The cell-related components, synergistic components, and optional other components are mixed evenly; 3), if necessary, the mixture is divided according to the volume ratio of the drug volume / target volume required for the therapeutic effect and cover; 4), if necessary, steri...
Embodiment approach
[0111] 1. The use of non-pathogenic cell-related components as active ingredients that can provide therapeutic effects in the preparation of locally administrable pharmaceutical compositions for treating local lesions.
[0112] 2. The application according to item 1, wherein the pharmaceutical composition further comprises one or more chemically active ingredients or / and biologically active ingredients capable of producing synergistic effects with the non-pathogenic cell-related components, and in the In the above pharmaceutical composition, the ratio of the cell-related component to the chemically active component (cell-related component concentration / chemically active component concentration) is (1-110) / (1-100) or (1.5-100 ) / (0.1-1), or / and the ratio of the cell-related component to the biologically active component (cell-related component concentration / bioactive component concentration) is (60-180) / (0.1-60) .
[0113] 3. A topically administrable pharmaceutical composition...
Embodiment 1
[0186] Embodiment 1: the preparation of composition
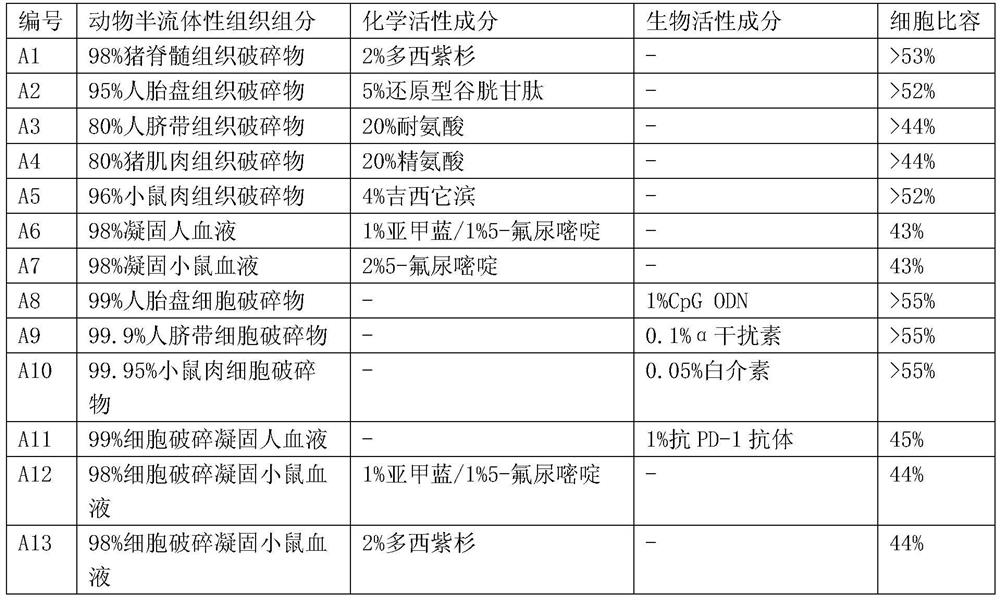
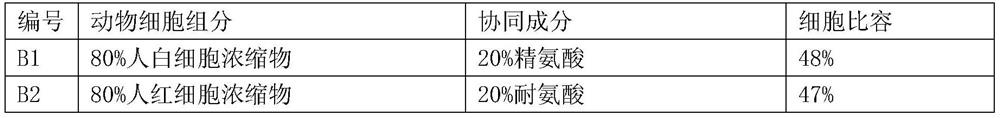
[0187] Numerous different compositions of the present invention can be formulated according to the preparation methods of the compositions of the present invention described above. The compositions of some compositions of the present invention prepared in this example are listed in Table 2 (the cell-related components are prepared from animal tissues), Table 3 (the cell-related components are prepared from animal cells) and Table 4 (the cell-related components are prepared from prebiotic bacteria preparation). The cell specific volume listed in the table, wherein the cell specific volume of the tissue is provided by the veterinarian of the relevant animal experiment company, and the cell specific volume of the blood and cell concentrate is determined according to the method of blood routine measurement (for example, using the automatic blood cell analyzer BC 5000) .
[0188] Table 2
[0189]
[0190] Several examples ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com