Methods of treating ocular neovascular diseases using AAV2 variants encoding aflibercept
A new blood vessel, disease technology used in the treatment of ocular neovascular diseases using AAV2 variants encoding aflibercept to address vision loss, increased intraocular pressure, retinal detachment, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0326] Example 1: In 6×10 11 An open-label phase 1 study of AAV2.7m8-aflibercept in neovascular (wet) age-related macular degeneration at vg / eye dose
[0327] This example describes the use of AAV2.7m8-aflibercept, an rAAV vector containing the VEGF inhibitor aflibercept and the AAV2.7m8 protein capsid, for the treatment of age-related macular degeneration (AMD) with choroidal neovascularization ) (wet AMD; wAMD) open-label phase 1 study.
[0328] I. Purpose of the study
[0329] A. Main purpose
[0330] The primary objective of this study was to evaluate the safety and tolerability of a single intravitreal (IVT) injection of AAV2.7m8-aflibercept in subjects with wAMD.
[0331] primary endpoint
[0332] The primary endpoint of the study was the type, severity and incidence of ocular and systemic adverse events (AEs).
[0333] B. Secondary purpose
[0334] The secondary objectives of these studies are to:
[0335] · To evaluate the effect of AAV2.7m8-aflibercept o...
Embodiment 2
[0552] Example 2: In Neovascular (Wet) Age-Related Macular Degeneration Below 6×10 11 Dose vg / eye and open-label phase 1 study of AAV2.7m8-aflibercept with topical corticosteroids
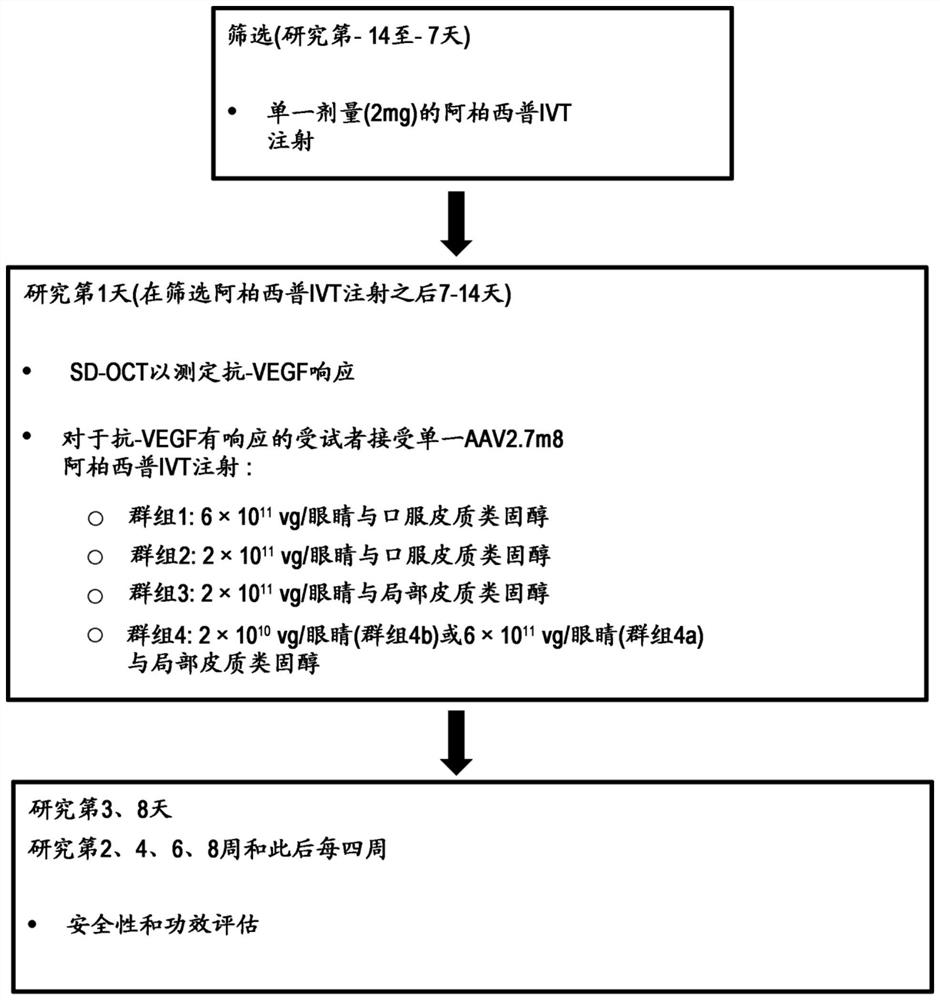
[0553] The following examples describe a continuation of the Phase 1 study described in Example 1 to evaluate 11 Dose of vg / eye and safety and efficacy of AAV2.7m8-aflibercept administered with topical corticosteroids in subjects with wAMD.
[0554] I. Study Objectives and Endpoints
[0555] The primary objective, secondary objectives and primary and secondary endpoints are as described in Example 1, Part I.
[0556] II. Research object
[0557] The subjects of this study were as described in Example 1, Part II. III. Investigational Drugs
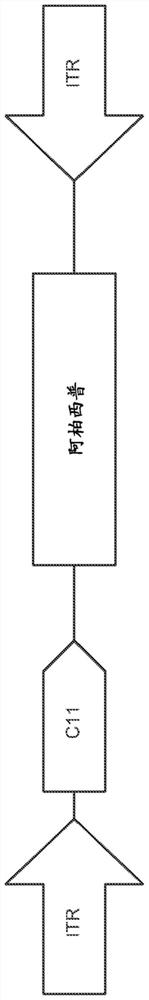
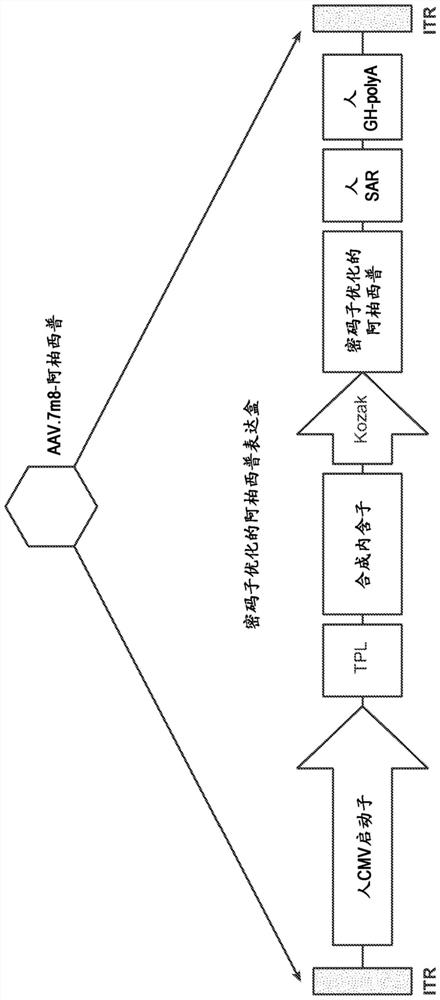
[0558] The study drug was AAV2.7m8-aflibercept as described in detail in Part III of Example 1 and as Figure 1A , except that the concentration of AAV2.7m8-aflibercept was varied in order to maintain an appropriate injection volume, as described in...
Embodiment 3
[0621] Example 3: In neovascular (wet) age-related macular degeneration, below 6×10 11 Results of an open-label phase 1 study of AAV2.7m8-aflibercept at a dose of vg / eye with topical corticosteroids
[0622] This example describes the results of the Phase 1 study described in Example 2, which evaluated subjects with wAMD at less than 6×10 11 Dose of vg / eye and safety and efficacy of AAV2.7m8-aflibercept administered with topical corticosteroids.
[0623] result
[0624] group 2
[0625] The baseline characteristics of the subjects in Cohort 2 are provided in Table 13.
[0626] Table 13. Baseline Characteristics of Subjects in Cohort 2
[0627]
[0628] As described in Example 2, subjects in cohort 2 received a 2×10 11 Dose 2 of vg / eye administered a single IVT injection of AAV2.7m8-aflibercept with 60 mg of prednisone starting 3 days before and 3 days after AAV2.7m8-aflibercept treatment for 6 days. This was followed by a 7-day prednisone taper. A summary of the...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap