Binding agents and uses thereof
A technology of association and inhibitor, applied in the field of binding agent and its application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
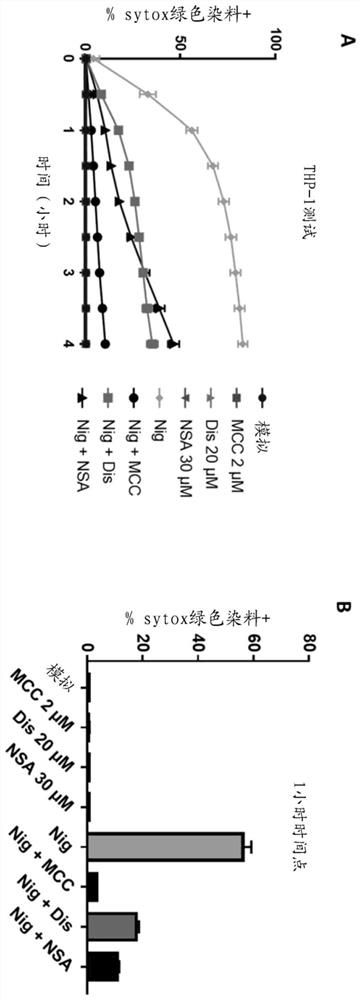
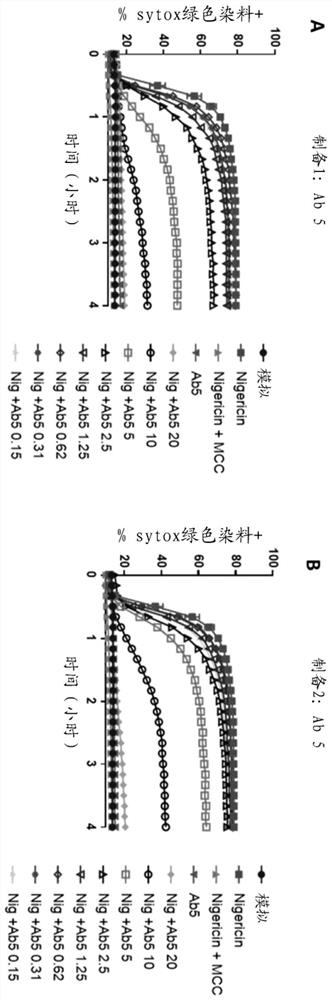
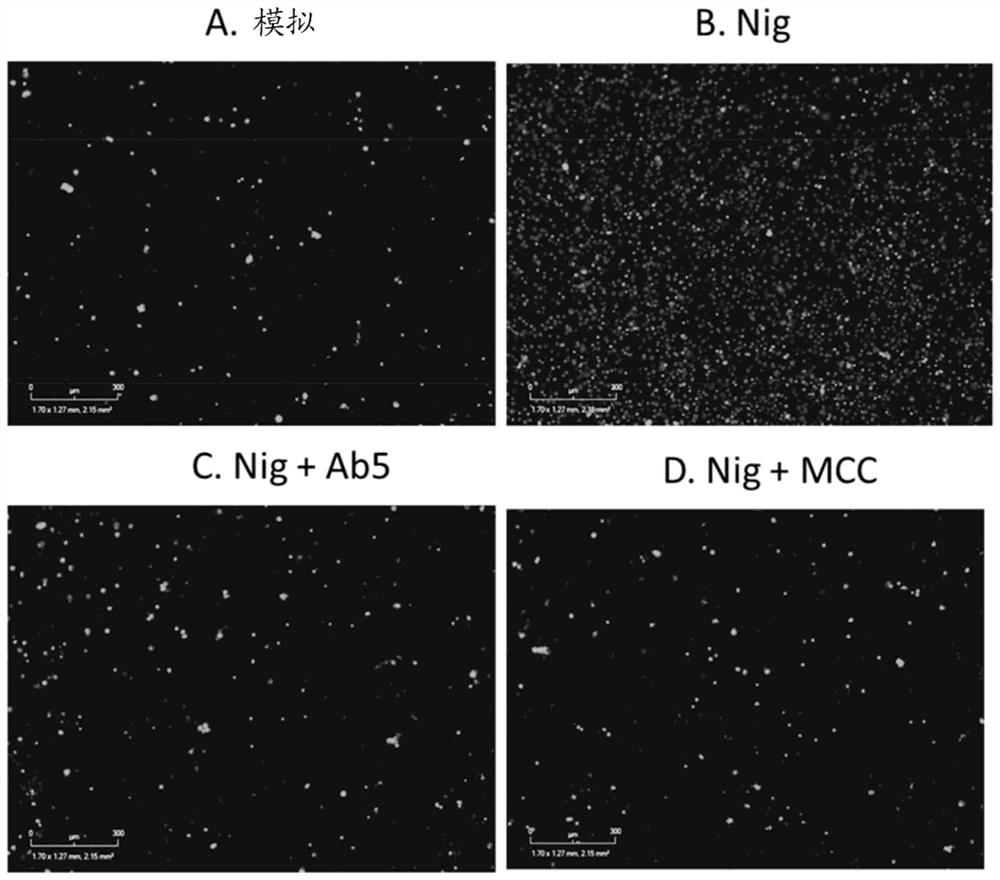
[0620] CLAIMS 1. An antidermatin D inhibitor that binds to and inhibits antidermatin D, such as wherein the inhibitor binds to and neutralizes antidermatin D.
[0621] 2. The antidermatin D inhibitor according to embodiment 1, wherein the inhibition of antidermatin D is the inhibition of:
[0622] i) the activity of nodermin D, such as wherein the inhibitor neutralizes the activity of nodermin D; and / or
[0623] ii) A function of nodermatin D, such as wherein the inhibitor neutralizes noderrmin D.
[0624] 3. An antidermatin D inhibitor that binds to and neutralizes antidermatin D.
[0625] 4. The antidermatin D inhibitor according to embodiment 3, wherein the neutralization of antidermatin D is neutralization as follows:
[0626] i) activity of nodermatin D; and / or
[0627] ii) Function of nodermatin D.
[0628] 5. The inhibitor according to any one of embodiments 1 to 4, wherein the inhibitor is an extracellular inhibitor.
[0629] 6. The inhibitor according to any one ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com