Synthesis method and application of chiral paramagnetic probe Py-D-Cys-DTPA
A technology of py-d-cys-dtpa and synthesis method is applied in the field of synthesis of chiral paramagnetic probe Py-D-Cys-DTPA, which can solve the problem that the rigidity of paramagnetic probe is affected by protein modification sites and the like , to achieve the effect of strong rigidity and great universality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] a) Synthesis of N-(2-bromoethyl) di-tert-butyl iminodiacetate, i.e. the synthesis of compound 3;
[0047] 2-Bromoethylamine hydrobromide (20.5g, 100mmol) was dissolved in 100mL of DMF, and 100mL of tert-butyl 2-bromoacetate (30g, 150mmol) in DMF was added with stirring at room temperature, followed by DIPEA (70mL, 400mmol). The reaction was stirred at room temperature for 9-10 hours, filtered, concentrated, and passed through a column to obtain 23 g of the target compound 3 as a colorless oil with a yield of about 67%. 1 HNMR (400MHz, CDCl 3 )δppm: 3.46(s, 4H), 3.42(t, J=7.6, 2H), 3.12(t, J=7.6Hz, 2H), 1.45(s, 18H).
[0048] b) Synthesis of S-trityl-D-cysteine tert-butyl ester, i.e. the synthesis of compound 5;
[0049] S-trityl-D-cysteine (6.0 g, 16.5 mmol) was added to 100 mL of tert-butyl acetate, perchloric acid (4.8 mL, 70%) was added dropwise under ice cooling, and the reaction was stirred. After dropping, stir and react at room temperature for 1 to 2 hours...
Embodiment 2
[0056] Embodiment 2, application of paramagnetic probe Py-D-Cys-DTPA
[0057] 1) Quantitatively weigh the paramagnetic probe prepared in Example 1 (ie, compound 8), dissolve it in water, and prepare a 30 mM Py-D-Cys-DTPA aqueous solution, and the water used is MQ ultrapure water.
[0058] 2) 0.5 mL of 1.0 mM ubiquitin E18C, ubiquitin A28C, ubiquitin G47C and ubiquitin E64C four protein solutions were prepared respectively, and the buffer solution was selected as 20 mM Tris-Cl (pH=7.5).
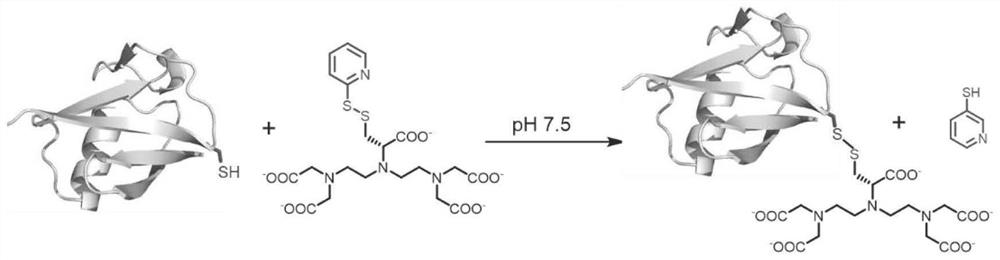
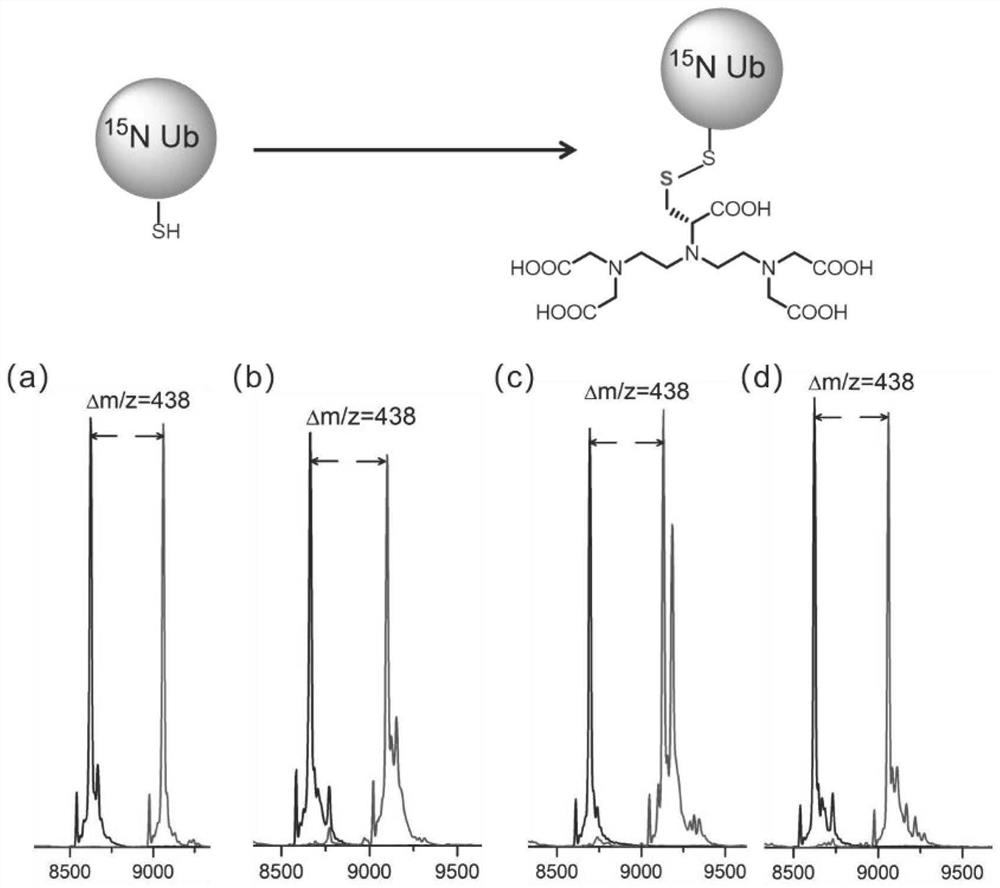
[0059] 3) Add 3 times the equivalent of the label to the prepared protein solution at room temperature, react for 3 hours, and mass spectrometry detects that the protein reaction is complete ( figure 2 ), and then purified with a PD10 column to obtain a site-specific modified protein ( figure 1 ).
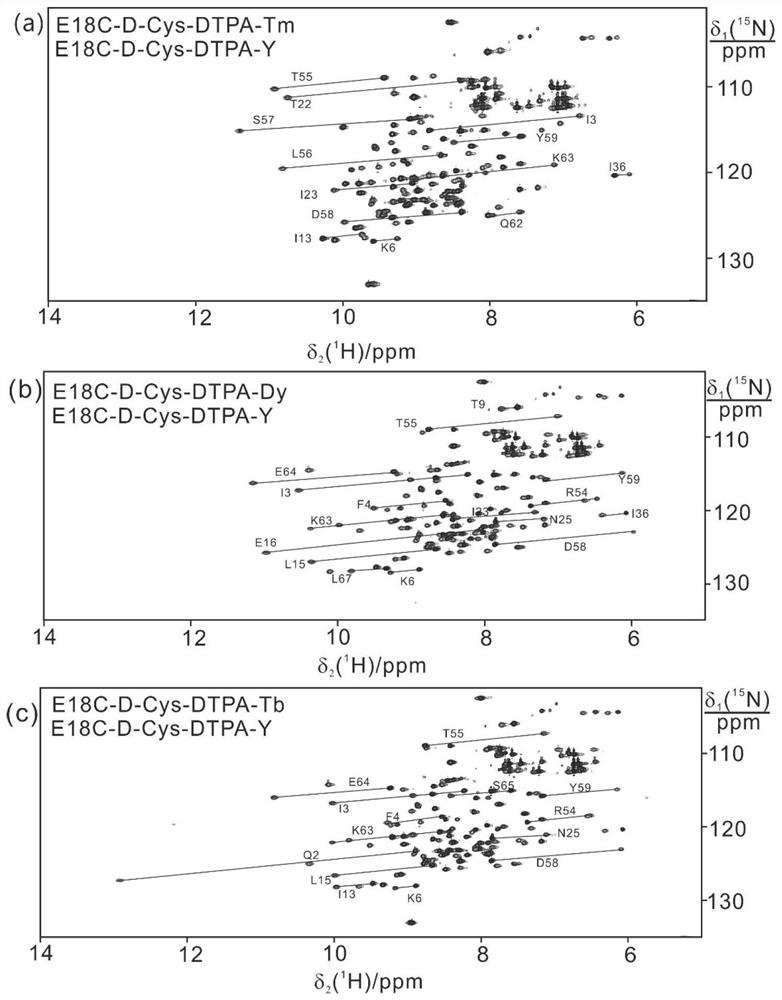
[0060] 4) Through the titration of rare earth ions, collect 1 H- 15 N HSQC spectrum ( image 3 ), the fitted tensor ( Figure 4 ).
[0061] All NMR experiments were collected on the Bruker-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com