Novel coronavirus vaccine antigen presentation system of attenuated salmonella secretory expression NTD structural domain protein and application of novel coronavirus vaccine antigen presentation system
A technology of domain protein and Salmonella, which is applied in the field of construction and preparation of recombinant attenuated Salmonella, can solve the problems of high efficiency, stability, and abnormally difficult secretion of antigen proteins, and achieves a strong price advantage, eliminating the need for adjuvant addition, and simplifying The effect of the operation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Construction of delivery plasmid for antigenic protein novel coronavirus protein NTD
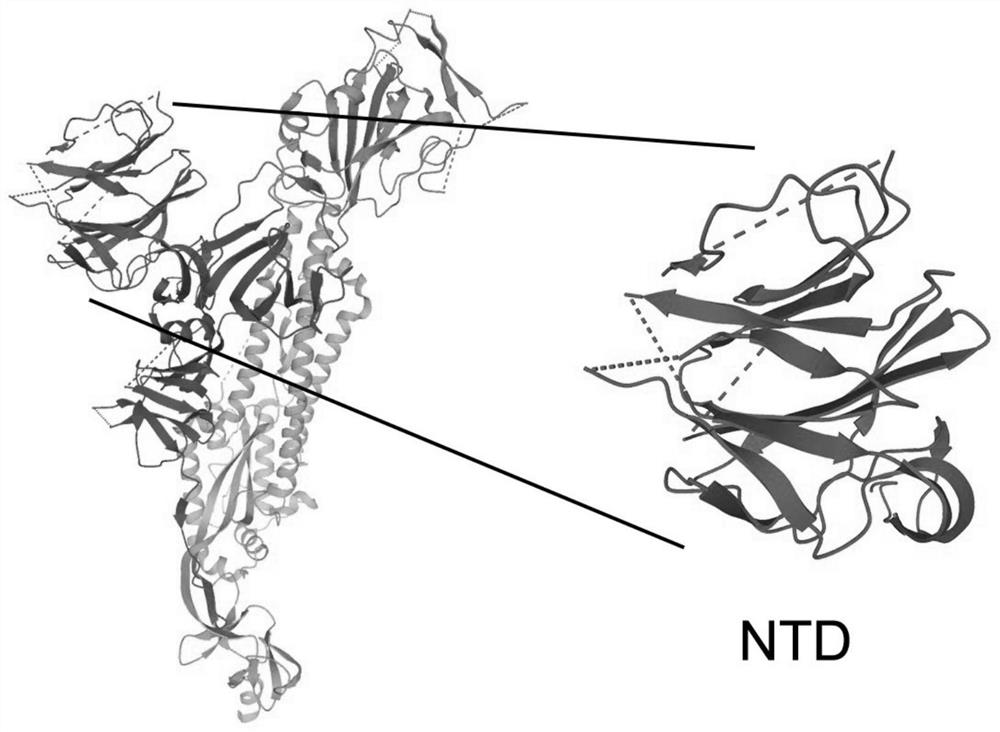
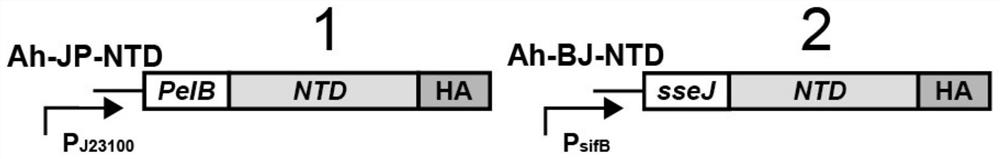
[0042] The NTD protein is a part of the structural region in the new coronavirus S protein ( figure 1 ), considering the large structural region of the S protein, after a large number of multi-angle bioinformatics analyzes and mutual comparisons, it was predicted that the NTD located at positions 13-303 of the overall amino acid sequence of the S protein contains more epitopes site. After a lot of experimental research, try various reported commonly used bacterial secretion systems, secretion signal peptides and matching promoters. In the constitutive expression secretion system Ah-JP-NTD plasmid, after screening, the present invention selects and uses a strong constitutive promoter J23100 and a pelB signal peptide. The gene sequence of the PJ23100-PelB is the nucleotide sequence shown in SEQ ID No.14.
[0043] For the inducible expression of type III secretory expression plasmids,...
Embodiment 2
[0061] Electroporation transformation of recombinant attenuated Salmonella:
[0062]Preparation of electroporation-competent Salmonella: Inoculate fresh attenuated Salmonella into 200mL LB medium, culture on a shaker at 37°C until the OD value is between 0.4-0.6, centrifuge at 5000rpm, collect the bacteria for 5min, and wash once with sterile double distilled water Finally, centrifuge at 5000rpm for 5min, wash the cells with sterilized 10% glycerol for 3-5 times, centrifuge at 5000rpm for 5min, resuspend with 500μL of 10% glycerol, aliquot 50μL / tube for electroporation. Transform the recombinant vaccine DNA vector into attenuated Salmonella by electroporation: under sterile conditions, add 0.5-5 μg of the constructed recombinant vector to the electroporation competent, mix well, and transfer to a 2mm electroporation cup, For electric shock, the electroporation conditions are 1.8kV, 25μF, 500Ω. After electroporation, spread on a kanamycin plate for screening, and the grown col...
Embodiment 3
[0064] Effective secretion detection of antigenic protein
[0065] The obtained recombinant attenuated Salmonella was cultured in kanamycin-resistant liquid LB medium to OD6000.8-1.0, the bacteria were collected and the OD600 value was adjusted to about 1.0 with PBS. Put it at 4 degrees for later use. The macrophage cell line RAW264.7 was induced with 100 ng / mL LPS to obtain M1 macrophages for 24 hours (hereinafter referred to as RAW264.7(M1)). The obtained RAW264.7(M1) was co-cultured with the Ah-BJ-NTD recombinant attenuated Salmonella obtained above at a ratio of 1:10 for 90 minutes, the supernatant was discarded and washed 2-3 times with PBS, and then added with 100ng / mL Qing The cells were cultured in damycin-based cell culture solution (10% serum, without double antibody) for 6 hours. Collect the cells and collect the total cell protein by thermal lysis, that is, resuspend the cells in 100 μL PBS, add 25 μL 5X Loading Buffer, and lyse at 100 degrees for 10-15 minutes. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com