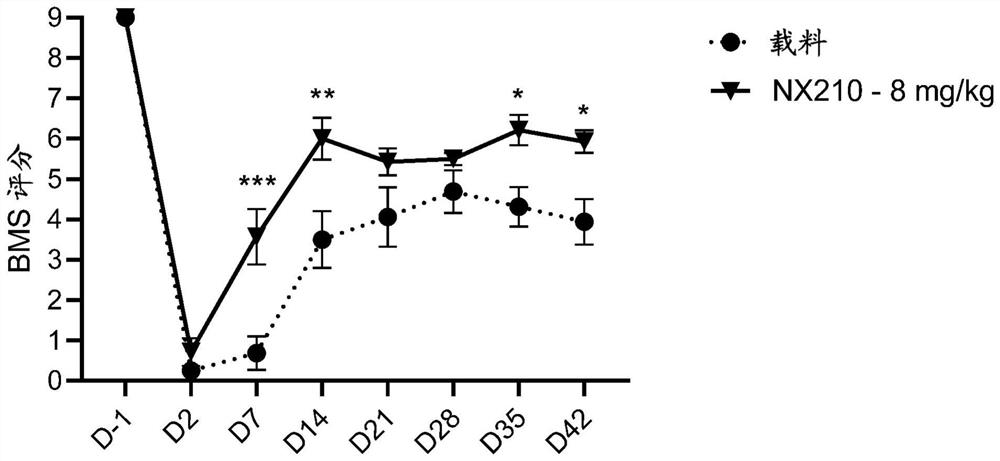
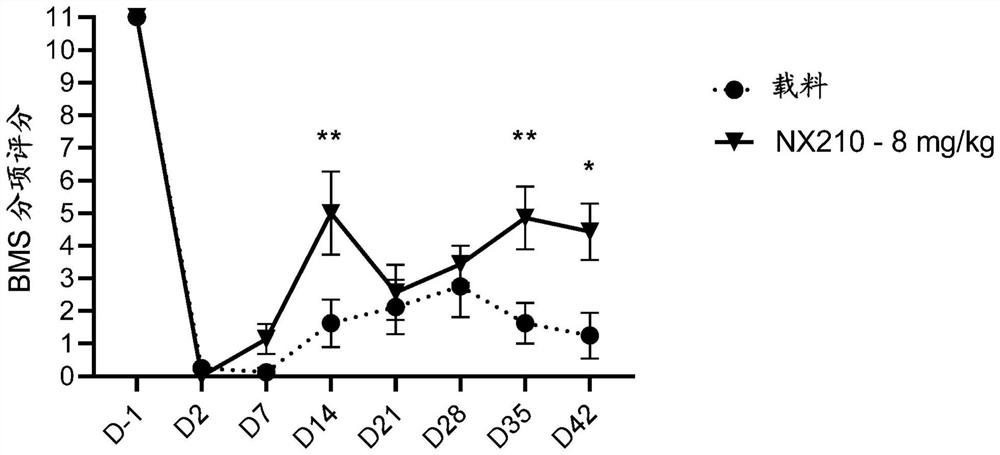
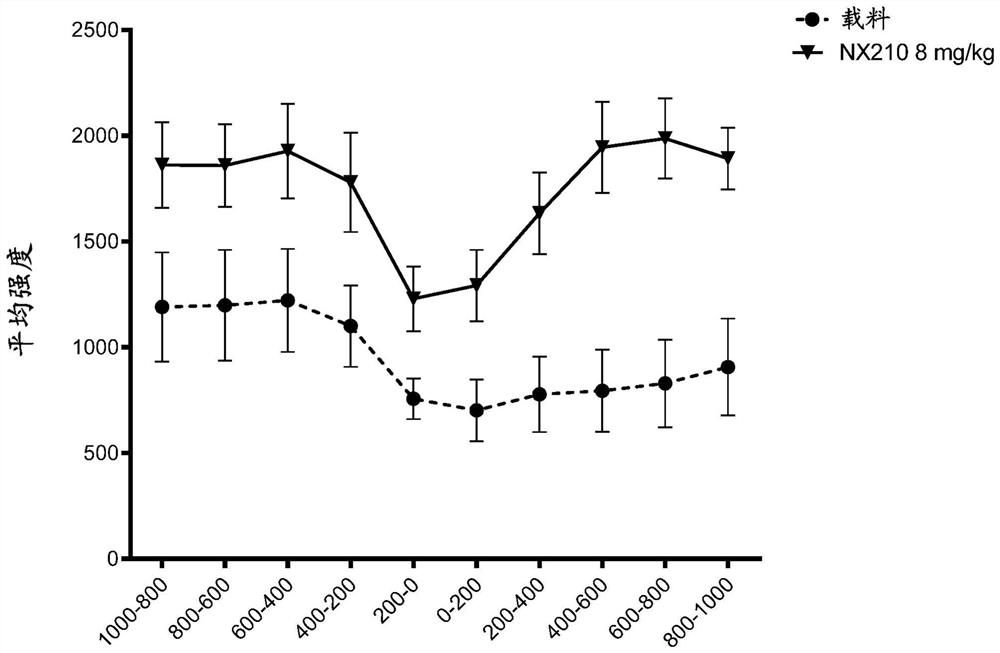
Systemic administration of peptides for treatment of spinal cord injury and/or remyelination
A spinal cord injury, systemic technology for systemic administration of peptides for the treatment of spinal cord injury and/or remyelination, capable of addressing issues such as low permeability, low bioavailability, and short half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0019] "SCO-spondin" is a central nervous system-specific glycoprotein found in all vertebrates from prechordates to humans. It's called an extracellular matrix molecule, and it's secreted by a specific organ located on top of the third ventricle (the subcommissural organ). It is a large size molecule. It consists of more than 4,500 amino acids and has a multi-module organization that includes various retained protein patterns, in particular including 26 TR or TSR patterns. Certain SCO-spondin-derived peptides starting from the TSR pattern are known to be biologically active in nerves or neural cells (described in particular in WO-99 / 03890).
[0020] A "TSR or TR pattern" is a protein domain of approximately 55-60 residues based on an alignment of the retained amino acids cysteine, tryptophan, and arginine. These patterns were first isolated in TSP-1 (thrombospondin 1), a molecule that interferes with blood clotting. They were then described in many other molecules such as ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com