Compositions and synergistic methods for treating infections
A composition and treatment effect technology, applied in the field of compositions for enhancing host immune defense, can solve the problems of lack of treatment options for treating pathogen infection, poor infection type, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0109] Preparation and Administration of Medication
[0110] The methods and compositions of the present invention have important implications for patient treatment and also for the clinical development of new treatments. It is also expected that clinical investigators will now use this method to determine entry criteria for human subjects in clinical trials. Healthcare practitioners choose treatment options based on the expected net benefit to the subject. The net benefit is derived from the risk-reward ratio.
[0111] The amount of treatment can be varied, eg, by increasing or decreasing the amount of gelsolin and / or antimicrobial agent administered to the subject, by varying the therapeutic composition administered, by varying the route of administration, by varying the timing of administration, and the like. The effective amount will vary depending on the particular infection or condition being treated, the age and physical condition of the subject being treated, the sev...
Embodiment 1
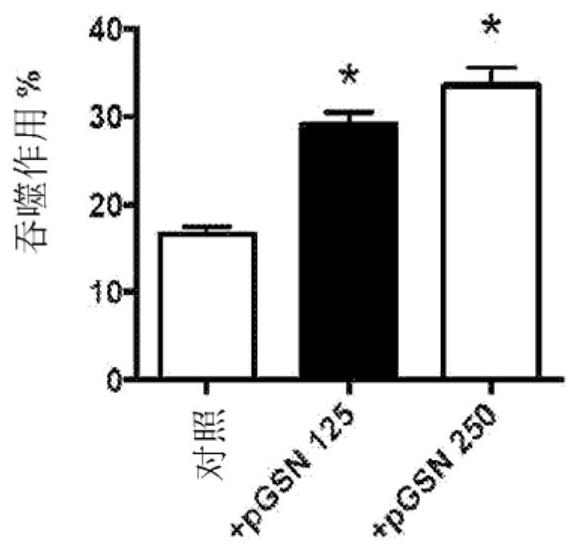
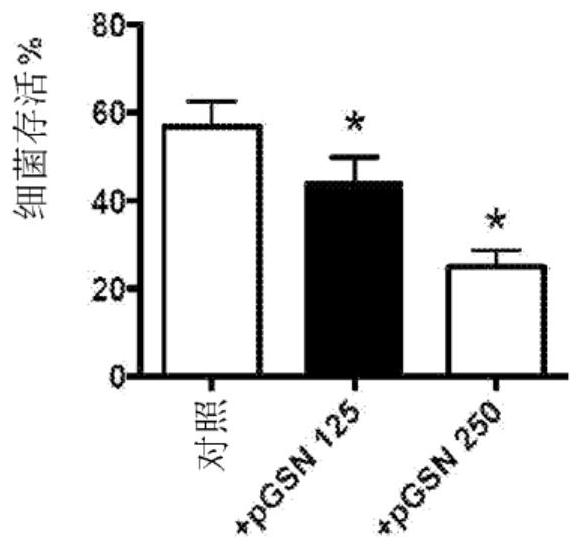
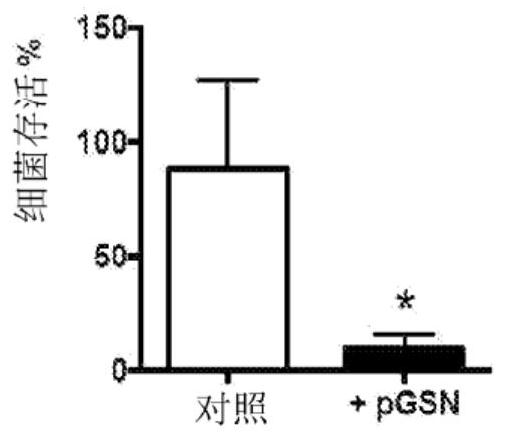
[0147] Antibiotic-resistant pneumococcal pneumonia occurs and can be a problem. Research has been conducted to evaluate new therapeutic strategies for fighting infections, including ways to enhance innate immunity. Experiments were performed to determine the effect of pGSN administration on macrophage and host survival.
[0148] method
[0149] Bacterial strains and cultures
[0150] Streptococcus pneumoniae serotype 3 (Cat. No. 6303, American Type Culture Collection, Rockville, MD) was grown overnight on agar plates supplemented with 5% sheep blood (Cat. No. 90001-282, VWR, West Chester, PA) and were prepared and quantified as previously reported (Yang Z. et al., Am J Physiol Lung Cell Mol Physiol 2015; 309:L11-6).
[0151] In vitro and in vivo procedures
[0152] (1) In vitro studies
[0153] In vitro studies were performed in which 125 to 250 μg / ml pGSN was added to bacterial cultures and bacterial survival was determined.
[0154] (2) In vivo studies
[0155] B16 mi...
Embodiment 2
[0161] Studies were conducted to evaluate the effect of pGSN treatment on antibiotic-sensitive and antibiotic-resistant mouse models of pneumococcal pneumonia.
[0162] method
[0163] Bacterial strains and cultures
[0164] S. pneumoniae serotypes 3 and 14 (catalog numbers 6303 and 700677, respectively) were obtained from the American Type Culture Collection (Rockville, MD). Serotype 3 bacteria were grown overnight on 5% sheep blood-supplemented agar dishes (Cat. No. 90001-282, VWR, West Chester, PA) and prepared and quantified as previously reported (YangZ. et al., Am J Physiol Lung Cell Mol Physiol 2015;309:L11-6). Because serotype 14 requires a more detailed protocol to achieve consistent results, the growth protocol reported in Restrepo AV et al., BMC Microbiol 2005;5:34 was performed using two consecutive Amplify and adjust bacterial concentration by OD600 for in vivo administration.
[0165] A mouse model of pneumococcal pneumonia
[0166] Normal 6 to 8 week (wk) o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com