Immune tolerant elastin-like recombinant peptides and methods of use thereof
A recombinant polypeptide and identity technology, applied in the direction of immunoglobulin, peptide/protein components, chemical instruments and methods, etc., can solve the problems of short antibody retention time, local immune checkpoint antibody treatment prospects are not as expected, high exposure, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0121] Example 1: Immunotolerant Elastin-Like Polypeptides (ITEPs) for Sustained Local Delivery of Immune Checkpoint Antibodies
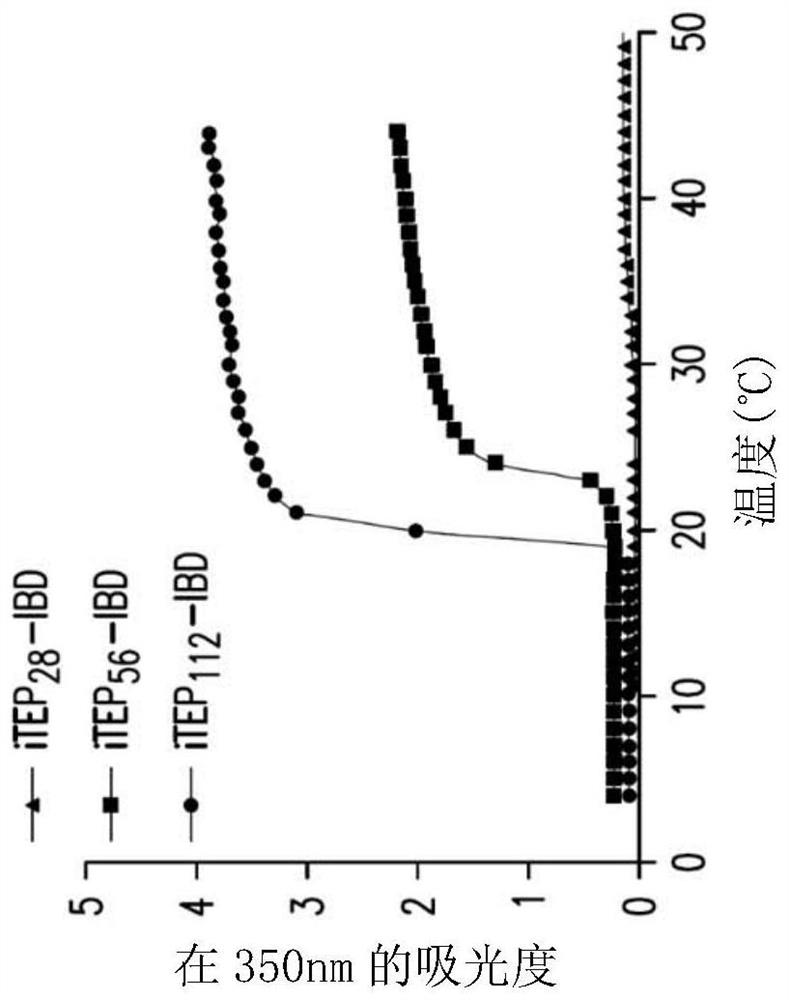
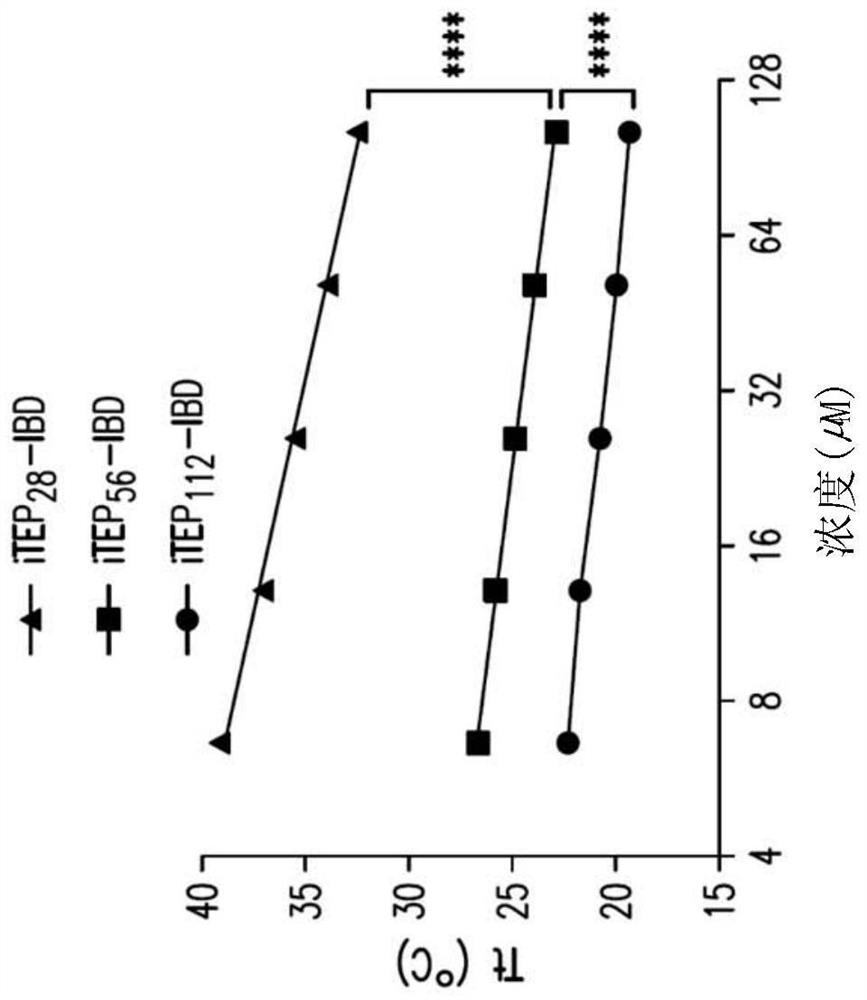
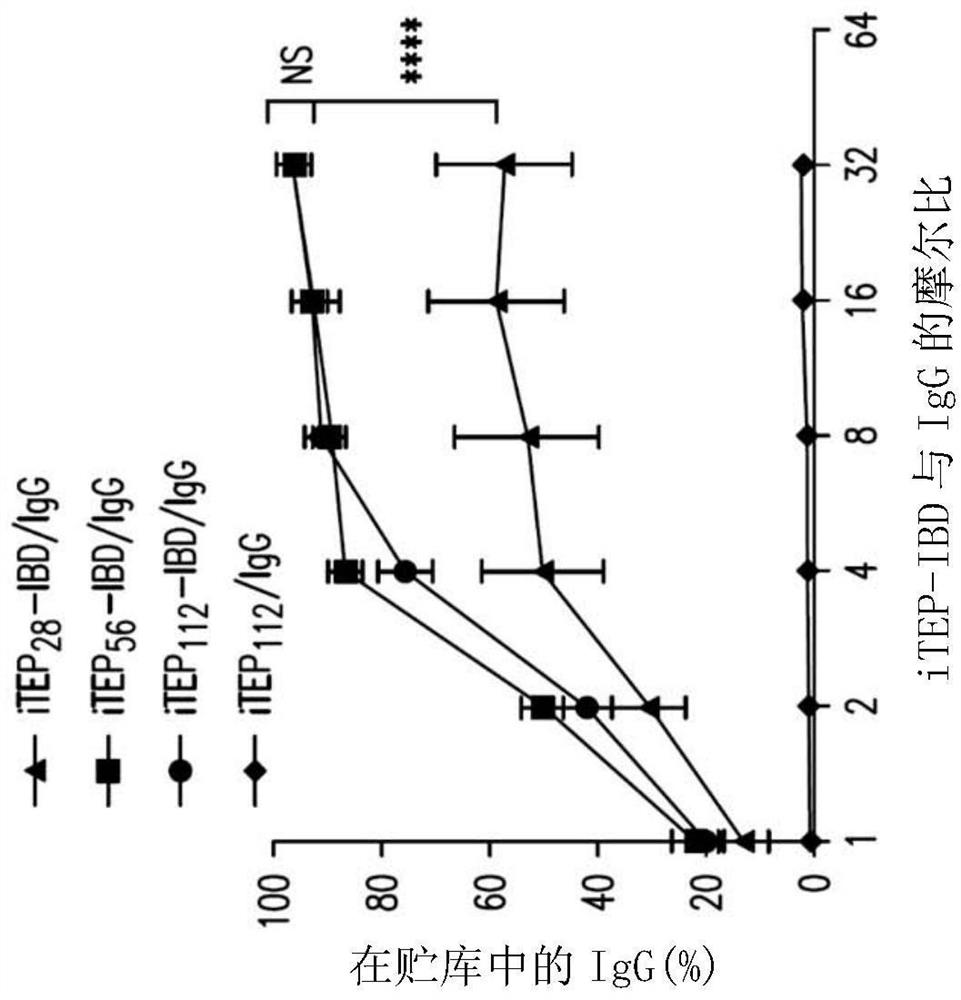
[0122] Abstract. To address the challenges associated with the systemic administration of immune checkpoint antibodies, immune-tolerant elastin-like polypeptide (iTEP)-based systems were developed to improve local delivery of immune checkpoint antibodies. Due to the phase-change nature of iTEP, the thermosensitive delivery system can form a sustained-release depot at the injection site. To link the antibody to the depot, the IgG binding domain (IBD) was fused to the iTEP. The results described herein demonstrate that iTEP-IBD polypeptides can prolong antibody release and increase antibody retention time at the local injection site. By controlling the design of the iTEP-IBD polypeptide, the release half-life of the antibody can be fine-tuned from about 17.2 to about 74.9 hours. Using melanoma as a disease model, the results showed that the iTEP-IBD...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap