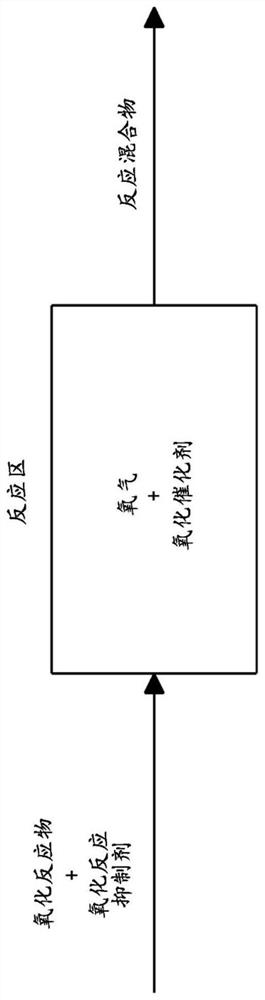
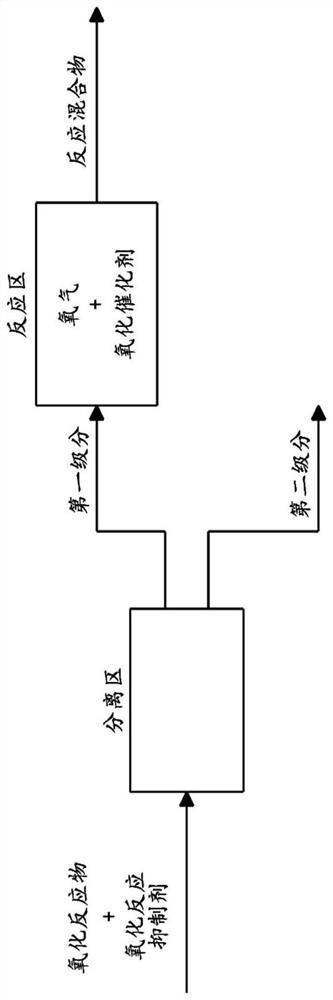
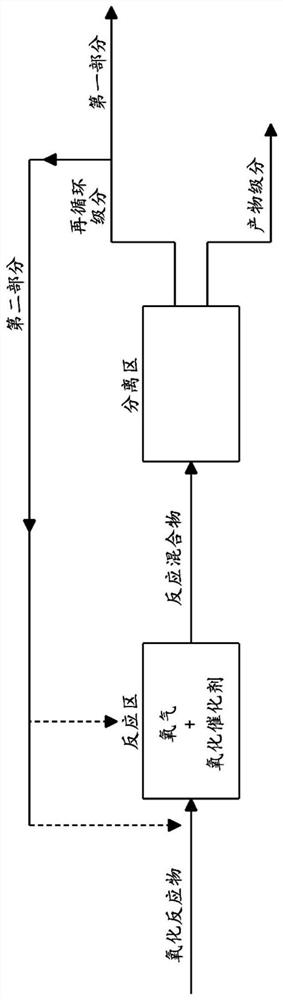
Method for preparing aldonic acid, aldonic acid and uronic acid
A technology of aldaric acid and uronic acid, which is applied in the field of preparation of aldaric acid, aldonic acid and uronic acid, and can solve the problems that effective solutions for reducing or eliminating inhibition have not yet been developed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0144] The following non-limiting examples are provided to further illustrate the present invention.
example 1
[0145] Example 1: Using Au / TiO 2 Catalyst converts glucose to gluconic acid
[0146] 1 wt.% Au / TiO 2 The catalyst is charged into a fixed packed bed reactor. A solution of 20wt.% glucose in water for 2.0hr -1 The liquid hourly space velocity (LHSV) of 50% air, 50% N was fed into the reactor in co-current flow at a rate of 1000 SCCM 2 air flow. The pressure of the system was maintained at 750 psig. The temperature of the reactor jacket was varied between 75°C and 85°C.
[0147] The reaction was carried out for about 1750 hours. Between 1520 and 1568 hours of operation, the feed was changed to include a 0.8 wt. % glyceric acid and 20 wt % glucose feed stream. like Figure 7 As shown in , glucose conversion and gluconic acid yield were significantly reduced when glyceric acid was present in the feed stream. Glucose conversion decreased from about 100% to about 35%, and gluconic acid yield decreased from about 98% to about 45%.
[0148] The reaction temperature also decr...
example 2
[0150] Example 2: Conversion of Gluconate to Glucarate Using Pt / C Catalyst
[0151] About 25 mg of 4 wt.% Pt / C catalyst was charged into the batch reactor. 2.3 mL of a 10 wt.% solution of gluconic acid in water was introduced into the reactor. The reactor was maintained at 85°C and pressurized to 1800 psi with air. The reaction was carried out for 1 hour.
[0152] Seven additional reactions were performed under the same conditions with various compositions introduced into the gluconic acid feed solution to test conversion inhibition. The gluconic acid feed solution was tested to contain 0.08M propionic acid, 0.08M lactic acid, 0.08M glyceric acid, 0.04M glyceric acid, 0.02M hydroxymalonic acid, 0.02M tartaric acid and 0.05M malic acid.
[0153] Figure 8 The conversion of gluconic acid and the production of guluronic acid, glucarate, 2-keto-gluconate, glycerate, and 5-keto-gluconate in each experiment are reported. Rate. Each experiment was performed in duplicate.
[01...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com