Novel chemical method for preparing 6-chloro-4-(4-fluoro-2-methylphenyl) pyridine-3-amine as key intermediate of NT-814
A kind of technology of methyl phenyl, methyl phenyl boronic acid, be used in preparation compound A, preparation compound IX or compound A, the application field in preparation compound A, can solve high cost, material cannot be obtained commercially, yield is low And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0234] In the following, embodiments of the present invention are disclosed. The first embodiment is denoted E1, the second embodiment is denoted E2, and so on.
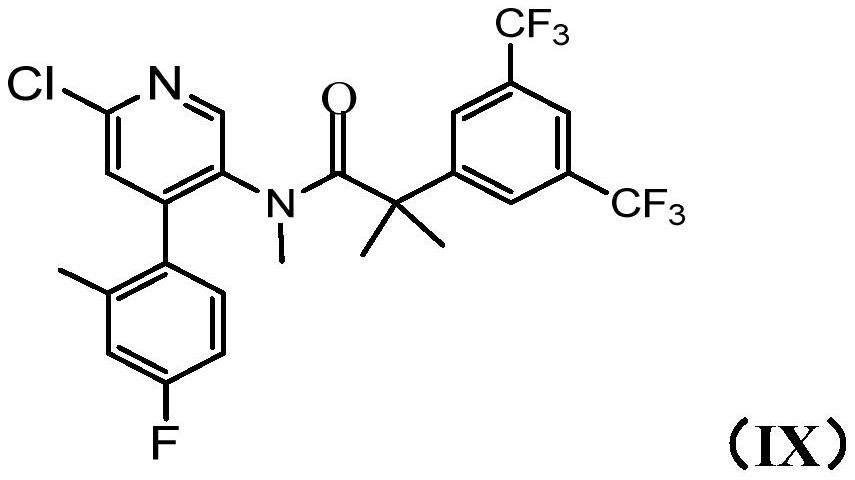
[0235] E1 A method for preparing compound IX:
[0236]
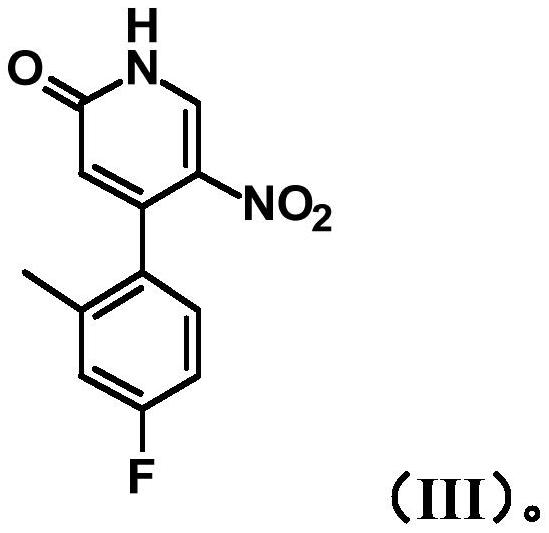
[0237] The method comprises the following steps i) bringing 4-chloro-5-nitropyridin-2(1H)-one (compound I) and 4-fluoro-2-methylphenylboronic acid (compound II) in the presence of a base The reaction is catalyzed by a palladium complex to provide 4-(4-fluoro-2-methylphenyl)-5-nitropyridin-2(1H)-ones of structure (III):
[0238]
[0239] E2 The method of embodiment 1, wherein the palladium complex is selected from the group consisting of palladium acetate, tetrakis(triphenylphosphine)palladium, bis(triphenylphosphine)palladium(II) dichloride, and [1,1 '-bis(diphenylphosphino)ferrocene]dichloride palladium(II), phosphine palladium complex, preferably tetrakis(triphenylphosphine) palladium.
[0240] E3 The method of any one of Embodiments 1-2, wherein the...
Embodiment 1
[0324] 4-(4-Fluoro -2 Preparation of -methylphenyl)-5-nitropyridin-2(1H)-one (compound III)
[0325]
[0326] 4-Chloro-5-nitropyridin-2(1H)-one (39.0 g, 223 mmol, available from Leapchem), (4-fluoro-2-methylphenyl)boronic acid (39.6 g, 257 mmol; available for example from commercially available from Sigma Aldrich), potassium carbonate (92.6 g, 670 mmol), and 1,4-dioxane (480 mL) were charged into a three-necked flask connected to a condenser and vacuum / nitrogen line. After being evacuated and backfilled with nitrogen 3 times, tetrakis(triphenylphosphine)palladium(0) (12.9 g, 11.2 mmol) was added under nitrogen flow and the resulting mixture was stirred at gentle reflux (99°C internal temperature) for 18 hours. The reaction mixture was cooled to room temperature and filtered. The precipitate was washed with dioxane (300 mL) and the filtrate was discarded. The precipitate was then washed with MeOH (2 x 200 mL), the filtrate was collected, concentrated in vacuo and dried...
Embodiment 2
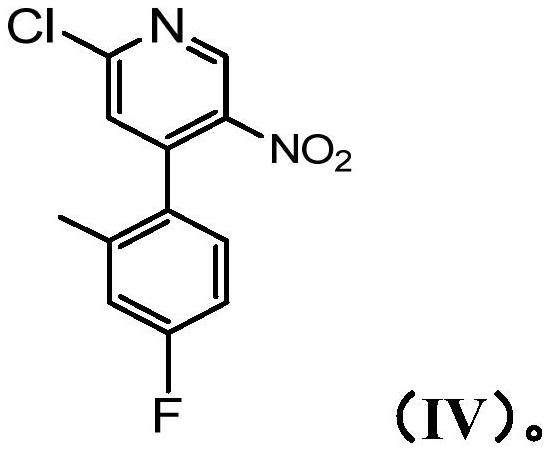
[0328] Preparation of 2-chloro-4-(4-fluoro-2-methylphenyl)-5-nitropyridine (Compound IV)
[0329]
[0330] 4-(4-Fluoro-2-methylphenyl)-5-nitropyridin-2(1H)-one (78.0 g, 60% w / w, 0.19 mol) was suspended in a three-necked flask under nitrogen in DME (600 mL). Add POCl dropwise 3 (0.10 kg, 61 mL, 0.65 mol) (reaction was slightly exothermic, temperature rose to 40°C), then DMF (14 g, 15 mL, 0.19 mol) was added. The resulting mixture was stirred at 70°C (internal temperature) for 18 hours. The reaction mixture was cooled to room temperature and poured slowly into water (600 mL) (exothermic, cooled with ice / water bath). with solid Na 2 CO 3 The pH was neutralized and the mixture was transferred to a separatory funnel and extracted with EtOAc (2 x 600 mL). The organic layer was collected, washed with brine, washed with Na 2 SO 4 Drying, filtration and concentration in vacuo gave the title compound (46.2 g, 173 mmol, 91% w / w yield) as a brown solid in 86% purity (HPLC). ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com