Compounds and methods for modulation of estroglen receptors
A technology for compounds and compositions, applied in the directions of active ingredients of heterocyclic compounds, drug combinations, organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
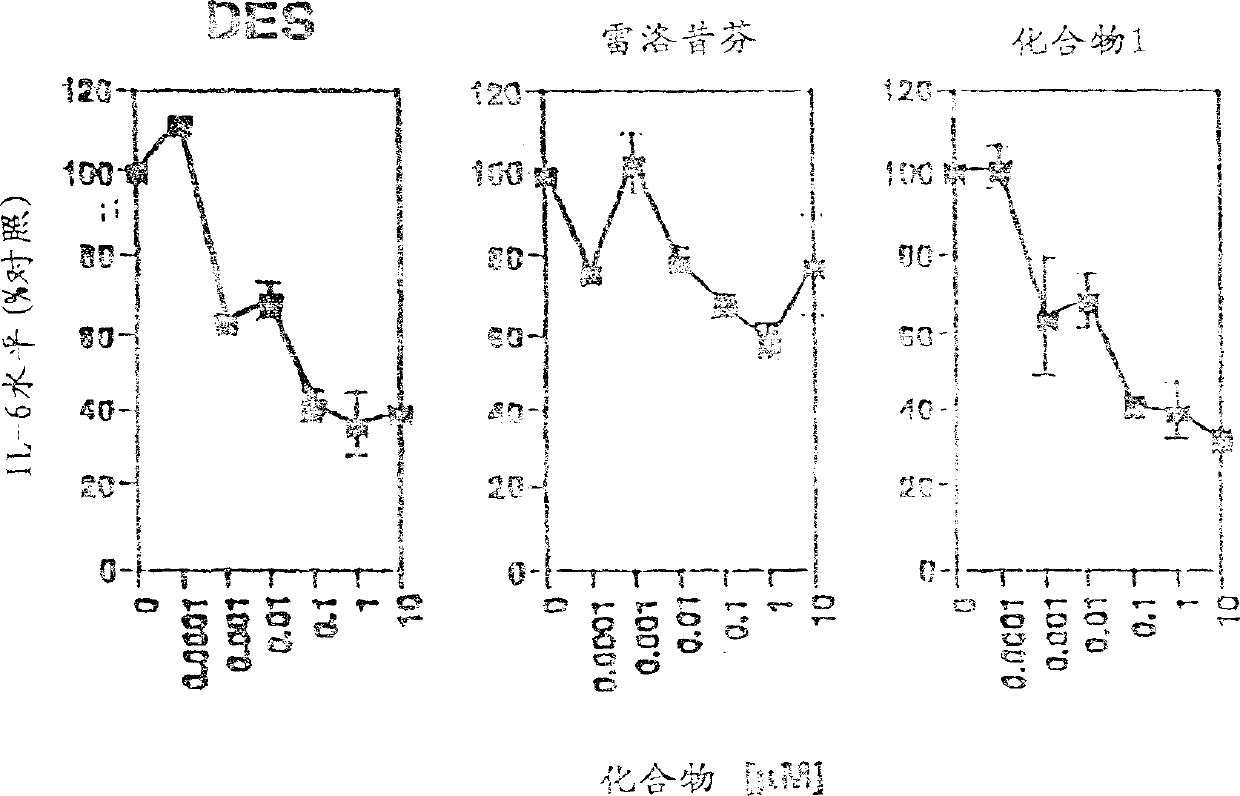
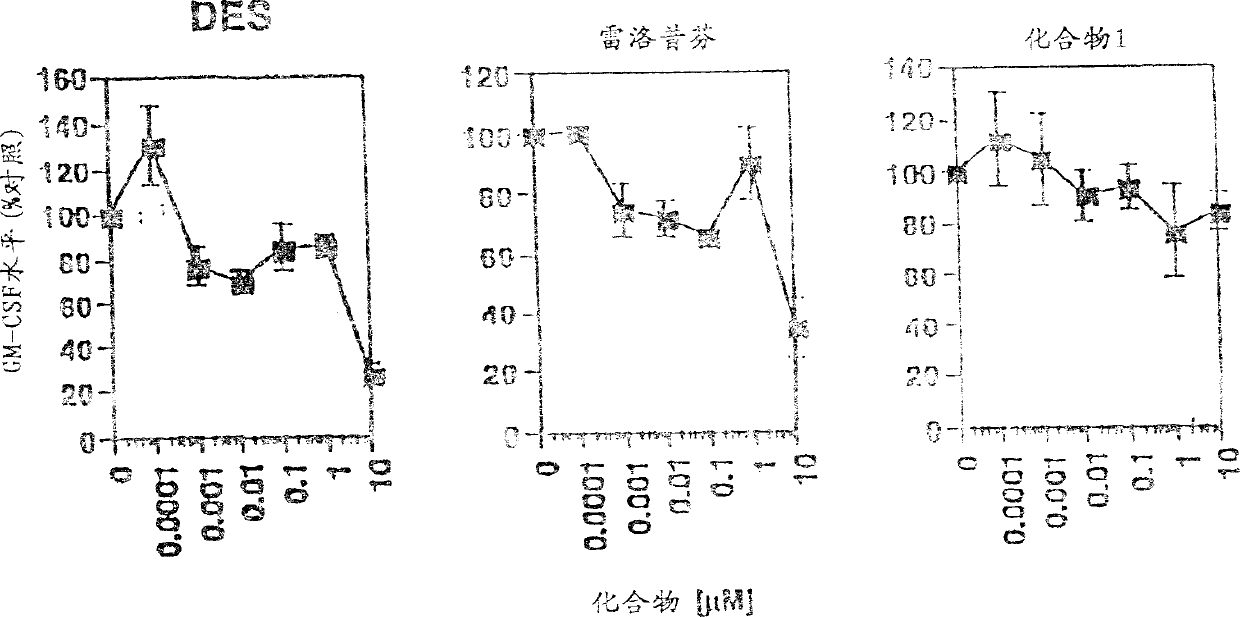
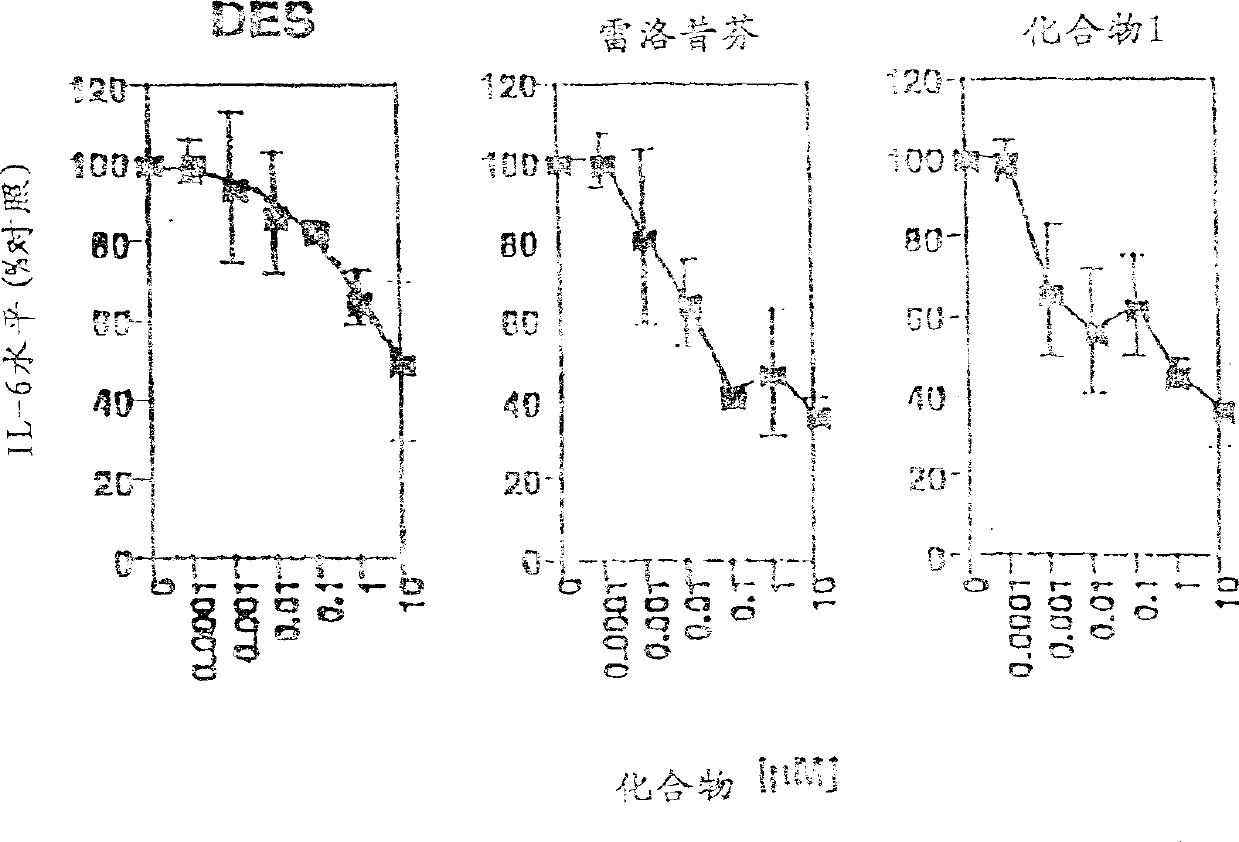
Image
Examples
Embodiment 1
[0133] 2-(4-Hydroxybenzylacetone)-5-methoxyphenol
[0134]
[0135] To 3-methoxyphenol (50g, 0.40mol), 4-hydroxyphenylacetic acid (71g, 0.46mol) and ZnCl 2 (174g, 1.28mol) was added with POCl 3 (100ml, 1.6mol). The mixture was stirred at 65°C for 2 hours, poured into ice water (2 L) and stirred until the ice melted. The clear supernatant was decanted, the residue was rinsed with water (1 L), and partitioned between EtOAc and water. The organic layer was washed with brine, dried (MgSO 4 ), filtered and concentrated. The resulting oil was purified by chromatography (SiO 2 , 20% EtOAc / n-Hexane) provided 2-(4-hydroxybenzylacetone)-5-methoxyphenol (34.1 g, 33% yield) as a white solid; mp 137-140°C.
Embodiment 2
[0137] 2-(4-Triisopropylsilyloxybenzylacetone)-5-methoxyphenol
[0138]
[0139] To 2-(4-hydroxybenzylacetone)-5-methoxyphenol (10g, 0.038mole), NEt 3 (6ml, 0.042mole) in CH 2 Cl 2 (50ml) was added triisopropylsilyl chloride (9ml, 0.042mole). The mixture was stirred for 22 hours, concentrated, and the residue was dissolved in EtOAc and H 2 O distribution. The organic layer was washed with NaOH (1N), HCI (1N) and brine. The organic layer was dried (MgSO 4 ), filtered and concentrated. The residue was treated with n-hexane to give 2-(4-triisopropylsilyloxybenzylacetone)-5-methoxyphenol (6.2 g, 38% yield) as a white solid; mp 66-68°C .
Embodiment 3
[0141] 3-Phenyl-4-(4-hydroxybenzyl)-7-methoxycoumarin
[0142]
[0143] To 2-(4-triisopropylsilyloxybenzylacetone)-5-methoxyphenol (4g, 9.6mmole), K 2 CO 3 (4g, 29mmoles) in CH 3 To the mixture in CN (50ml) was added phenylacetyl chloride (2.3ml, 14mmole). The mixture was stirred at reflux for 22 h, poured into H 2 O (0°C) (500ml), extracted with EtOAc (2x). The organic layer was dried (MgSO 4 ), filtered and concentrated. Residue and Et 2 With O stirring, the resulting solid was filtered and recrystallized (EtOH) to give 3-phenyl-4-(4-hydroxybenzyl)-7-methoxycoumarin (0.88 g, 15% yield) as a white solid; mp 235-236°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com