Compositions, reagents and kits for and methods of diagnosing, monitoring and treating hormonal imbalance
A technology for hormones and biological samples, applied in chemical instruments and methods, biochemical equipment and methods, hormone peptides, etc., can solve problems such as no function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0173] Example 1 - Separation
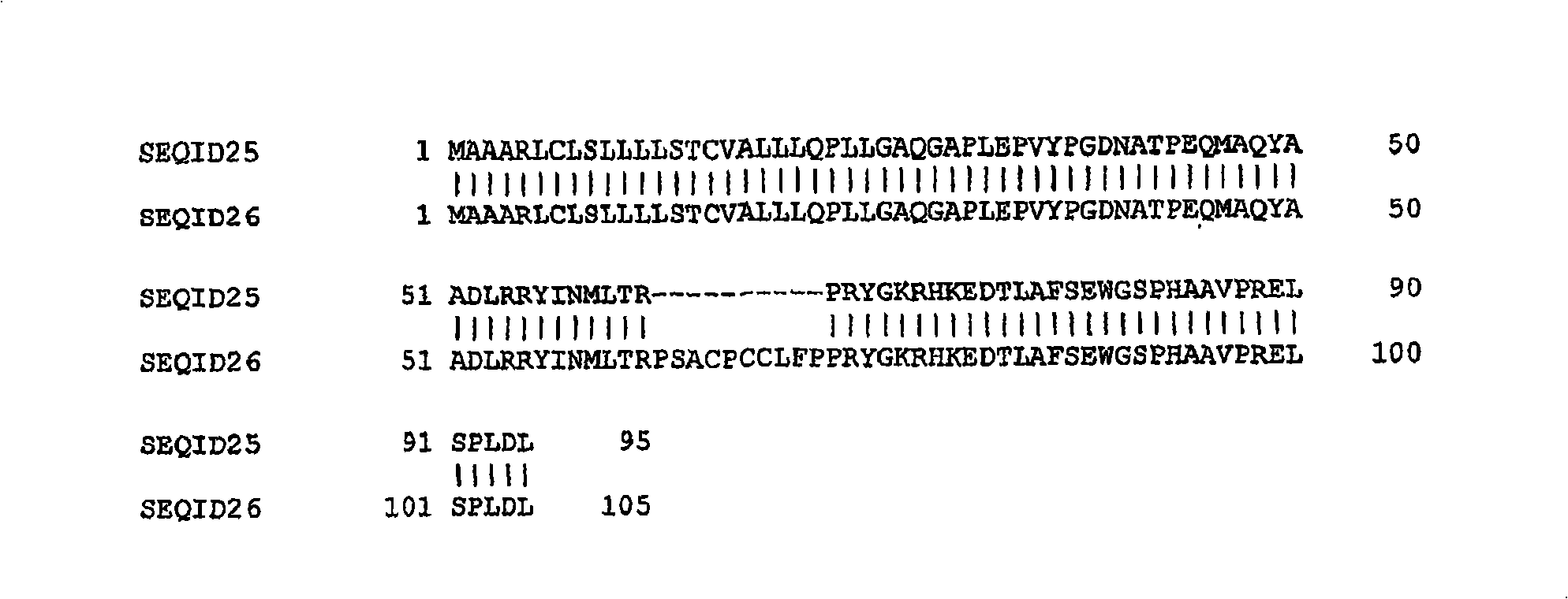
[0174] With an MOI equal to 2, with expression comprising SEQ ID NO: 21 or SEQ ID NO: 23-SEQ ID NO: 24 or SEQ ID NO: 26 or SEQ ID NO: 28 or SEQ ID NO: 30 or SEQ ID NO: 32 Or the baculovirus of the amino acid sequence of SEQ ID NO: 34 or of SEQ ID NO: 36 or of SEQ ID NO: 38 infects Sf-9 cells with a hormone-dysregulated variant (AC-hormone-dysregulated variant). Cells were grown at 28°C with continuous shaking (90 rpm). At 60 hours post infection (hpi), the medium was collected and cells were separated from the medium by centrifugation at 5000 rpm for 5 minutes. 10 ml of the culture medium were separated by cation exchange chromatography using an SP-Sepharose column. The column was equilibrated with PBS pH 6.5, and after loading the sample onto the column, the column was washed with PBS to elute unbound protein (flow-through fraction). Elution was performed with increasing concentrations of NaCl at a flow rate of 2 mL / min (5% NaCl / min).
[...
Embodiment 2
[0176] Example 2 - Secretion
[0177] Sf-9 cells were infected with a baculovirus expressing a hormone dysregulated variant (Ac-hormone dysregulated variant) at an MOI equal to 2. Cells were grown at 28°C with continuous shaking (90 rpm) and 1 mL samples were collected at 24, 48 and 60 hours post infection (hpi). After centrifugation, cell pellets were lysed with lysis buffer (50 mM Tris pH 7.5, 1% tritonX100, and protease inhibitor cocktail) at 4°C for 30 minutes and sonicated for 30 seconds. The samples were centrifuged at 14000 rpm for 10 minutes and the supernatant was designated as Pellet. The sample buffer was added to 40 μL of the Pellet preparation and 40 μL of medium (specified medium), and electrophoresed on 15% SDS-PAGE. After electrophoresis, the gel was subjected to semi-dry protein transfer to a nitrocellulose membrane. The membranes were incubated for 2 hours with anti-hormone dysregulation variant antibodies and for an additional 1 hour with secondary anti...
Embodiment 4
[0181] Example 4 - Synthetic Production of Hormone Disorder Splice Variant Analogous Compounds
[0182] Amino acid derivatives and synthetic reagents are commercially available. Peptide chain extension can be performed using Applied Biosystem 433A synthesizer produced by Perkin Elmer, and the protected peptide derivative-resin can be constructed by the Boc or Fmoc method. The protected peptide resin obtained by the Boc method was deprotected with anhydrous hydrogen fluoride (HF) in the presence of p-cresol to release the peptide and then purified. The protected peptide resin obtained by the Fmoc method was deprotected with trifluoroacetic acid (TFA) or diluted TFA containing various scavengers, and the released peptide was purified. Purification was performed by reverse phase HPLC on a C4 or C18 column. The purity of the purified product can be confirmed by reverse-phase HPLC, and its structure can be confirmed by amino acid composition analysis and mass spectrometry.
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com