Potentiation of anti-CD38-immunotoxin cytotoxicity
A technique of immunotoxin, toxin, applied in the fields of immunology and tumor biology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
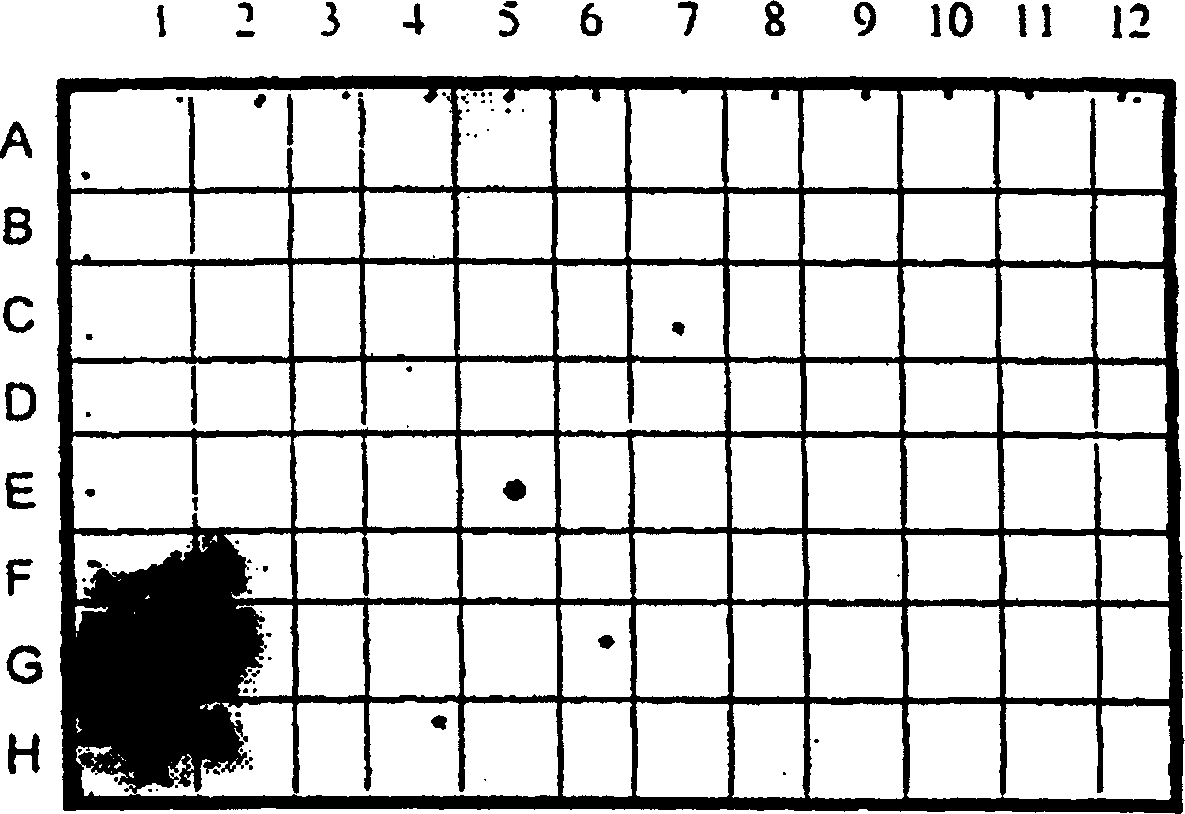
[0030] CD38 expression in normal tissues is largely restricted to the thymus
[0031] The tissue specificity of CD38 was detected by dot blot hybridization of radiolabeled CD38 nucleic acid probe to commercial (CLONTECH) tissue specific mRNA. figure 1 Hybridization results are shown. CD38 was observed to be predominantly expressed in the thymus, with significantly lower levels in the prostate.
Embodiment 2
[0033] Retinoic acid (RA) enhances the cytotoxic effect of immunotoxins by increasing the expression of CD38.
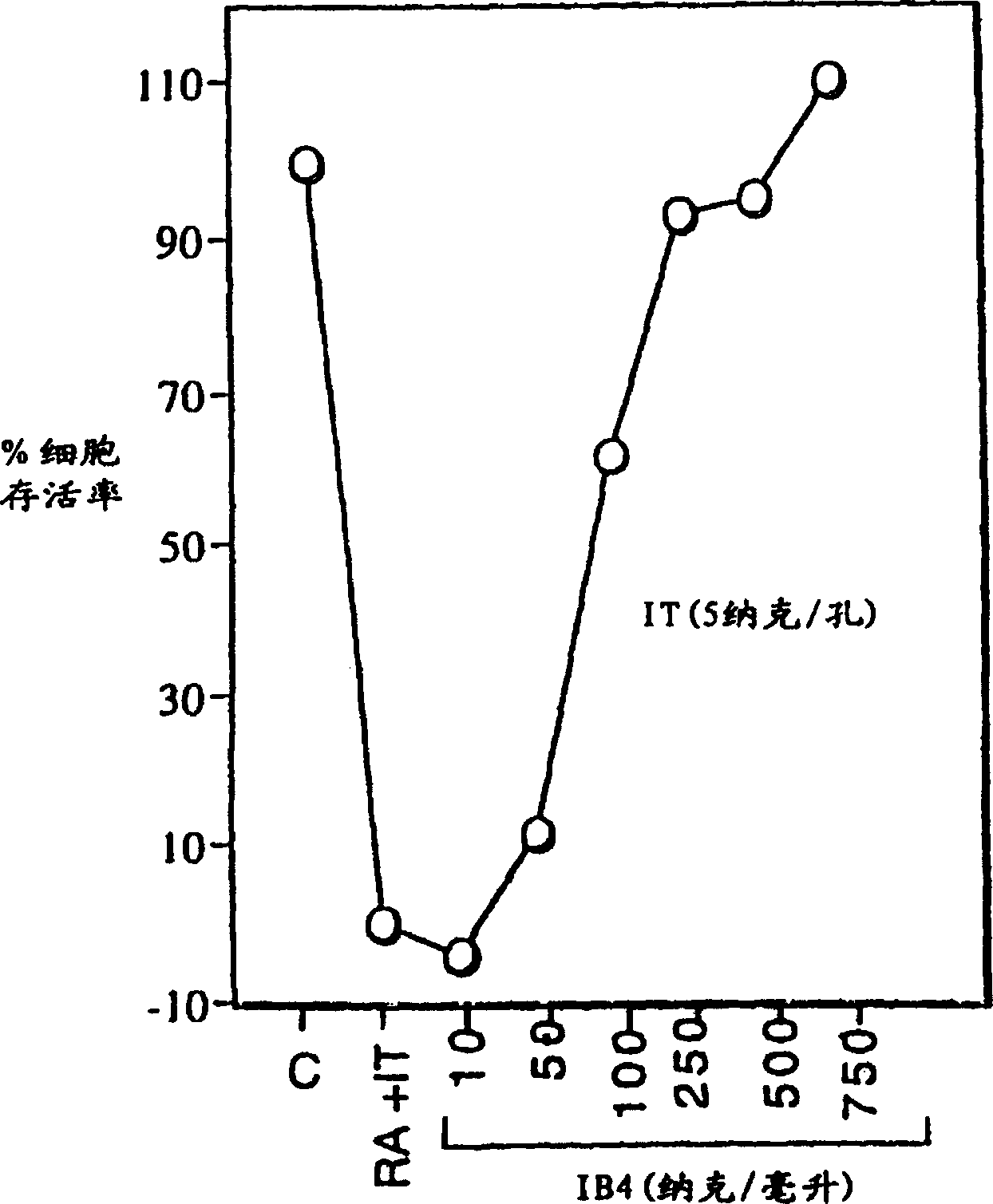
[0034] HL-60 cells were incubated with immunotoxin alone or in the presence of 5 nM retinoic acid (RA). Increasing concentrations of unconjugated IB4 monoclonal antibody were added to cells incubated with immunotoxin and retinoic acid. After 3 days, cell viability was measured by MTS assay. Briefly, a 6.5 mg / ml solution of MTS [(3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-( 4-thiophenyl)-2H-tetrazolium] and 0.5mM PMS (phenazine methanesulfonate) solution.The mixed MTS / PMS solution of 20 microliters is placed in the sample containing the cells to be tested in the 96-well plate In each well, incubate the plate for 1-4 hours at 37°C in a 5% CO2 atmosphere, and then measure the amount of formazan produced by MTS cell reduction of live cells by reading the absorbance at 490 nm. figure 2 The result is shown.
[0035] Immunotoxin alone had minimal effec...
Embodiment 3
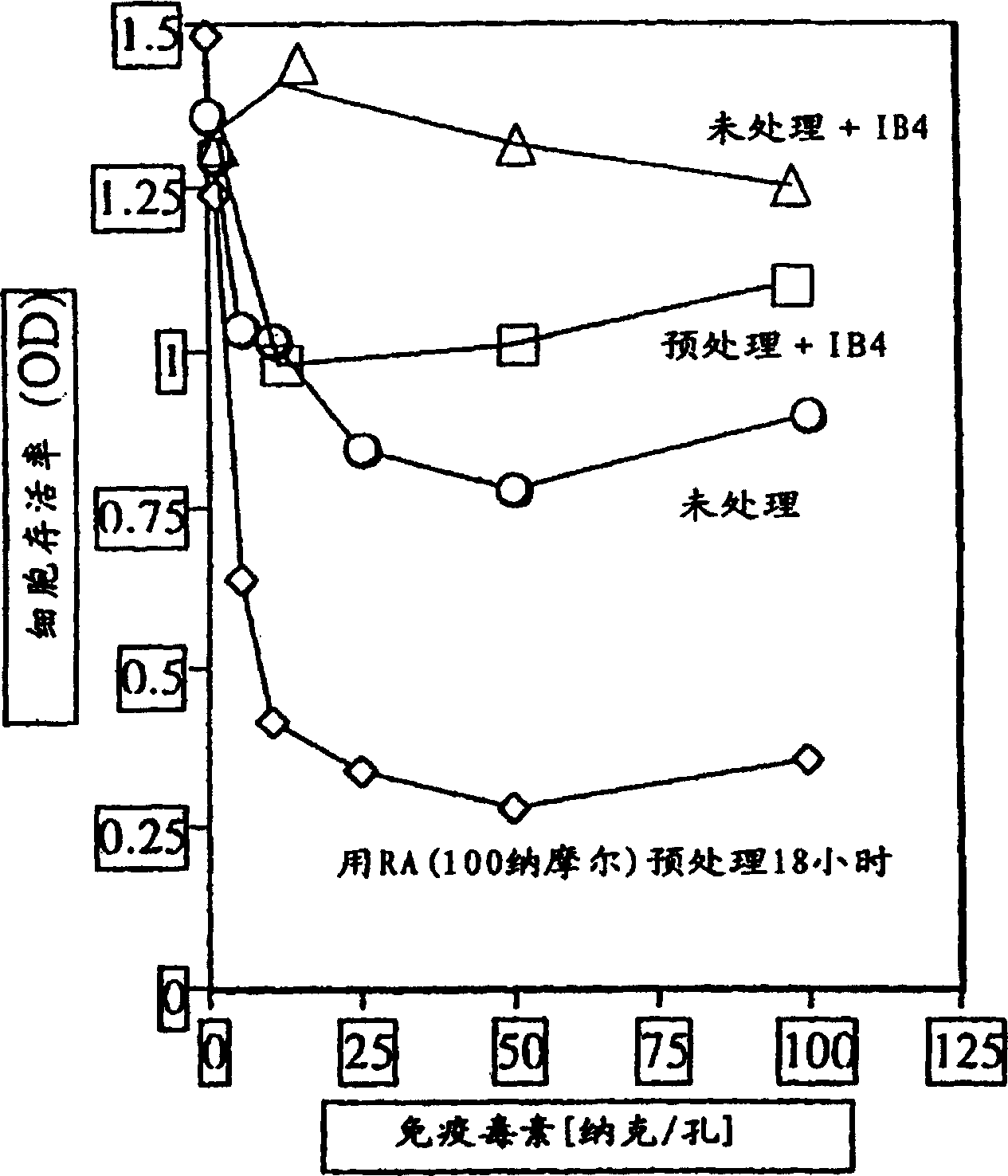
[0037] Pretreatment with all-trans retinoic acid (RA) enhanced the induced killing of HL-60 cells.
[0038] HL-60 cells were preincubated overnight in the presence or absence of 5 nM all-trans retinoic acid. Cells were washed twice and incubated in increasing concentrations of immunotoxin in the presence or absence of IB4 unconjugated anti-CD38 monoclonal antibody. After 3 days, cell viability was tested. image 3 The result is shown.
[0039] Preincubation with all-trans retinoic acid followed by immunotoxin treatment resulted in more cell death than immunotoxin treatment alone. A 100-fold excess of unconjugated anti-CD38 mAb IB4 blocked the toxicity of the immunotoxin in both cases by competing with the immunotoxin and binding of the CD38 marker on cells. These results suggest that all-trans retinoic acid (RA) causes changes in cells that make them more susceptible to immunotoxins, rather than playing a direct role in target cell death.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com